Abstract
Short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) are generated from paired-pulse transcranial magnetic stimulations (ppTMS) using certain interstimulus intervals (ISIs). ppTMS provides an accessible technique to evaluate inhibitory/facilitatory motor neural circuits. However, SICI and ICF are highly variable such that individual variability is not captured by any one static ISI. We hypothesized that individuals may have individualized and relatively stable pattern of SICI/ICF profiles. We tested SICI and ICF profiles using ISIs from 1 to 500 ms, on 2 occasions about 3 weeks apart, and the test-retest reliability, in 23 healthy controls. Moderate-to-good test-retest reliabilities were found at ppTMS with 1 and 3 ms ISIs (SICI) and with 12, 15, 18 and 21 ms ISIs (ICF), but not with other control ISIs. A similar pattern of results was obtained for males and females. Interestingly, the peak facilitation, peak inhibition and maximum inhibition/facilitation ranges were individualized, such that they varied considerably across individuals but had high repeatability within individual (Cronbach’s alpha = 0.76 to 0.85). Therefore, individuals appear to have unique inhibition/facilitation profiles that are relatively stable. Although the functional implications of individualized profiles are currently unknown, the relatively stable profiles may index underlying neural inhibition and excitation traits.
Keywords: ICF, ICI, Paired-pulse, TMS
Introduction
Transcranial magnetic stimulation (TMS) of the primary motor cortex can elicit motor evoked potentials (MEPs) in the contralateral target muscles. The amplitudes and latencies of MEPs are reliable, but indirect, measures of pyramidal tract excitability as well as its cortico-cortical and cortico-subcortical connections (Rossini & Rossi, 2007). In the paired-pulse TMS paradigm (ppTMS), two magnetic stimuli are delivered in close sequence to the same cortical region through the same stimulation coil (Kujirai et al., 1993). The first conditioning stimulus (CS) modifies the response to the following test stimulus (TS). The effects of ppTMS depend on several parameters, e.g., the intensity of the CS, the intensity of the TS and the interval between the two stimuli (ISI) (Ilic et al., 2002). If both CS and TS are delivered at suprathreshold intensities with an ISI of 50 to 200 ms, the first pulse can inhibit the amplitude of the second pulse which is known as long-interval intracortical inhibition (LICI) (McClintock, Freitas, Oberman, Lisanby, & Pascual-Leone, 2011). If the intensity of CS and TS are close to the resting motor threshold (RMT) (Tokimura, Ridding, Tokimura, Amassian, & Rothwell, 1996), or CS is clearly above the RMT and TS is below or around the RMT (Ziemann, Tergau, Wassermann, Wischer, Hildebrandt, & Paulus, 1998; Hanajima et al., 2002), then facilitation (short-interval intracortical facilitation, SICF) from CS to TS can occur at discrete ISIs of about 1 – 1.5, 2.5 – 3.0 and 4.0 – 4.5 ms. When the intensities of CS and TS are set to subthreshold and suprathreshold, respectively, then both inhibition and facilitation can be elicited with the effects highly dependent on the ISI: the amplitudes of test MEPs are inhibited at short ISIs (SICI; 1 – 6 ms) and facilitated at longer ISIs (ICF; 9 – 25 ms) (Kujirai et al., 1993; Maeda, Gangitano, Thall, & Pascual-Leone, 2002; McClintock, Freitas, Oberman, Lisanby, & Pascual-Leone, 2011; Rosenkranz & Rothwell, 2003; Russmann, Lamy, Shamim, Meunier, & Hallett, 2009; Saisanen et al., 2011; Udupa, Ni, Gunraj, & Chen, 2010). This study focused on examining the effect of ISI using a fixed subthreshold-CS and suprathreshold-TS across 1 to 500 ms ISI range.
Abnormal SICI and/or ICF over motor cortex were observed in some neuropsychiatric disorders such as epilepsy (e.g., Badawy, Curatolo, Newton, Berkovic, & Macdonell, 2007; Rossini & Rossi, 2007), Parkinson’s disease (Chu, Wagle-Shukla, Gunraj, Lang, & Chen, 2009; Hanajima et al., 2001; Ridding, Inzelberg, & Rothwell, 1995), Alzheimer’s disease (Liepert, Bar, Meske, & Weiller, 2001; Olazarán, Prieto, Cruz, & Esteban, 2010), Tourette’s syndrome (Gilbert et al., 2005; Orth, Amann, Robertson, & Rothwell, 2005; Orth, Münchau, & Rothwell, 2008), and schizophrenia (Daskalakis, Christensen, Fitzgerald, & Chen, 2002; Liu, Fitzgerald, Daigle, Chen, & Daskalakis, 2009; Wobrock et al., 2009; Wobrock et al., 2008; Wobrock et al., 2010). Further, application of ppTMS over brain regions besides motor cortex, e.g., parietal cortex, can be used to indicate the existence of inhibitory and excitatory interactions with certain cognitive functions (Koch, Franca, Albrecht, Caltagirone, & Rothwell, 2006; Oliveri et al., 2000a; Oliveri et al., 2000b).
However, the power of SICI and ICF to show behavioral and disease effects may be limited by the high variability of ppTMS data between subjects and/or sessions. Orth and colleagues (2003) found considerable inter-subject variance at SICI with 2 ms ISI and ICF with 15 ms ISI. Further evidence from a large database indicated that there were subjects showing inhibition and others showing facilitation at both SICI (3 and 4 ms) and ICF ISIs (10 and 15 ms) (Wassermann, 2002). High inter-subject variability was also demonstrated in other studies (Boroojerdi et al., 2000; Maeda et al., 2002). Comparing SICI and ICF, SICI was more reliable than ICF such that most subjects would show inhibition at SICI ISIs (e.g., 2 ms), but for ICF ISI (e.g., 15 ms), a portion of subjects would show no facilitation or even inhibition (Orth, Snijders, & Rothwell, 2003). This may in part be due to individual differences in their underlying cortical neurophysiology.
The inter-session variability of SICI and ICF was assessed with various ISIs in some studies (Boroojerdi et al., 2000; Maeda et al., 2002). However, most did not use intraclass correlation coefficients (ICCs), a standard method to examine the test-retest reliability (Portney & Watkins, 2009; Rankin & Stokes, 1998). One recent study by Fleming and colleagues did use ICC to evaluate the reliability of SICI and ICF with 2.5 ms ISI for SICI and 12.5 ms ISI for ICF. They found high ICC (> 0.87) for SICI and poor ICC (< 0.30) for ICF (Fleming, Sorinola, Newham, Roberts-Lewis, & Bergmann, 2012) which is consistent with other studies (e.g., Orth et al., 2003). However, no study has systematically assessed the test-retest reliability of the ppTMS elicited SICI and ICF with various ISIs by using ICC. Importantly, potential implications of the large inter-subject variability have not been fully understood. In the present study, we applied ppTMS with ISIs from 1 to 500 ms to evaluate the reliability of the individual inhibition and facilitation profiles, aiming to test the hypothesis that there are reliable, individualized intracortical inhibition and facilitation functions.
Methods
Participants
Twenty-three healthy volunteers (age = 41.6 ± 13.9 years; 14 male and 9 female) participated in the study. All subjects were interviewed with the Structured Clinical Interview for DSM-IV (SCID) to exclude cases with DSM-IV Axis I diagnosis. Major medical and neurological illnesses, history of head injury with loss of consciousness, substance dependence within the past 6 months or substance abuse within 1 month (except nicotine or marijuana) were exclusionary. TMS screening interviews confirmed that none of the subjects had contraindications for TMS (Rossi, Hallett, Rossini, & Pascual-Leone, 2009). All subjects gave their written informed consent and the protocol was approved by the University of Maryland Baltimore Institutional Review Board.
Electromyography recording
Surface electromyography (EMG) was recorded from the right first dorsal interosseous (FDI) muscle with Ag/AgCl disc electrodes (CareFusion Inc., WI, USA) placed in a tendon-belly montage. A ground electrode was placed over the right ulnar styloid. The EMG signal was recorded in DC mode with NeuroScan synamp2 amplifier (Charlotte, NC), amplified (gain of 10) and band-pass filtered (10 to 100 Hz) with a 60 Hz notch filter, digitized at 1000 Hz and stored for offline analysis (Sommer, Classen, & Cohen, 2001). The offline analysis was conducted by using Scan 4.3 software (Neurosoft, Inc., EI Paso, TX) and MATLAB (MathWorks, Inc., Natick, MA). Peak-to-peak amplitude of the motor-evoked potentials (MEPs) was measured. Participants were evaluated in two sessions about 3 weeks apart.
TMS procedure
Focal magnetic stimuli were given through a figure-of-eight coil (70 mm outer diameter of each wing) using two Magstim 200 Magnetic stimulators with a monophasic current waveform connected to a BiStim module (Magstim Co., Whitland, UK). The coil was held by a mechanical arm with the coil handle pointing backward and rotated 45° away from the midline to induce currents that traveled in a posterior-to-anterior direction across the central sulcus (Brasil-Neto, McShane, Fuhr, Hallett, & Cohen, 1992; Kammer, Beck, Thielscher, Laubis-Herrmann, & Topka, 2001; Werhahn et al., 1994). Prior to the start of the experiment each subject underwent an anatomical MRI scan. These images were imported into Brainsight™ TMS Frameless Navigation system (Rogue Research Inc, Montreal, Canada) to allow for online control of coil positioning (Du, Chen, & Zhou, 2012). The stimulus target was the scalp position above the left motor cortex where TMS induced the maximum peak-to-peak MEP amplitude from the right FDI muscle on each session. The location used for the first session was marked in the Brainsight system and used for the second session. In the second session, we used the crosshair from the first session as the starting point for search until the maximum peak-to-peak MEP amplitude was identified, which could be on or slightly different from the location of the first session. During each session, one research assistant held the coil with the help of a mechanical arm, pressed and held the safety button during the test, visually checked the coil alignment using the crosshair display of the Brainsight and corrected position of the coil if necessary. The misalignment from coil to this original crosshair was kept less than 1 mm within each session. Only left motor cortex was tested, since inter-hemispheric variability of the paired-pulse curves is minimal in healthy subjects (Maeda et al., 2002; Maeda, Keenan, & Pascual-Leone, 2000, but see Civardi, Cavalli, Naldi, Varrasi, & Cantello, 2000). Subjects were instructed to remain relaxed throughout the application of TMS, while the muscle was monitored for relaxation through visual inspection of the EMG.
Motor threshold and paired pulse paradigm
Resting motor threshold (RMT) was defined according to conventional criteria as the minimum intensity needed to elicit a MEP of > 50 µV in at least 5 out of 10 consecutive stimuli (Rossini et al., 1994). RMT is reported as a percentage of the maximum stimulator output. The first subthreshold conditioning stimulus was at the intensity of 80% RMT and was confirmed not to induce MEPs in both our study and prior reports (Kujirai et al., 1993; Maeda et al., 2002; Orth et al., 2003). The intensity of the second stimulus was suprathreshold and set to 120% of RMT which has been reported for SICI and/or ICF (e.g., Garry & Thomson, 2009). In order to cover variable range of potential inhibitory and facilitatory ISIs, 14 ISIs were tested: 1, 3, 6, 9, 12, 15, 18, 21, 30, 40, 80, 120, 200 and 500 ms. Typically, SICI protocols include 1 and 3 ms ISIs to induce inhibition (Fisher, Nakamura, Bestmann, Rothwell, & Bostock, 2002; Roshan, Paradiso, & Chen, 2003; Vucic, Cheah, Krishnan, Burke, & Kiernan, 2009) while ICF protocols include 9 – 21 ms ISIs to induce facilitation (e.g. Saisanen et al., 2011; T. Wobrock et al., 2008). ISIs from 30 to 500 ms were rarely used (Nakamura, Kitagawa, Kawaguchi, & Tsuji, 1997) but were included to evaluate inhibition and facilitation over a wider range of ISIs as well as to identify potential inter-session variations. Single 120% RMT stimuli were delivered as a control condition (TS alone). A session included 6 trials for each ISI and 12 trials of TS alone. They were randomized and delivered in one session, with intertrial intervals that were also randomly assigned and jittered between 4 and 10 seconds. An in-house MATLAB task program was written to control the Bistim units to deliver all stimulus conditions in one sequence. Every subject received the same stimulus sequence twice separated by about 3 weeks. Seventeen subjects were tested at approximately same time of day across the two sessions. Six subjects were tested at different time of day across the two sessions. The signals for TMS stimuli and the MEP responses were simultaneously recorded onto the same file.
In order to evaluate whether the MEP physiological maximum is reached with a pulse intensity of 120% RMT, (i.e., ceiling effect), six of the 23 participants were re-tested with single TMS pulses at different intensities. Single pulse TMS was delivered randomly with 11 different intensities from 50% RMT to 150% RMT in 10% RMT step. Note that only 120% RMT and 150% RMT were compared to determine whether 120% RMT has reached the ceiling of EMG responses. Inter-trial intervals also ranged randomly from 4 to 9 seconds.
Data analysis
For ppTMS, the modulation of the CS on the following TS was expressed as response differential (MEPISI – MEPTS alone), such that negative values reflect inhibitory responses and positive values reflect facilitatory response in relationship to the response to the single test pulse. Repeated measures analysis of variance (ANOVA) was conducted to examine effects of ISI variation across the 2 sessions, where response differential of each ISI was the dependent variable and session (2 sessions) and ISI (14 ISIs) were within-subject factors. Greenhouse-Geisser corrections were applied for ANOVA when the assumption of sphericity was not met. Post-hoc comparisons were false discovery rate (FDR) corrected for multiple comparisons.
The variability of inhibition/facilitation across subjects was evaluated by examining the proportion of subjects that showed individual maximum facilitation and inhibition at each ISI.
The inter-session reliability was assessed for raw MEP amplitude and response differential using intraclass correlation coefficient (ICC) with a two way mixed effects model, based on the Shrout and Fleiss model (Fleming et al., 2012; Shrout & Fleiss, 1979). For the purpose of the current study, ICC above 0.6 was considered acceptable: between 0.6 – 0.8 moderate reliability and above 0.8 good reliability (Portney & Watkins, 2009).
As shown in the results, good repeatability of response differentials (ICC above 0.8) was only shown at SICI ISIs at 1 and 3 ms and ICF ISIs at 12 to 21 ms ISIs. Therefore, we calculated the following parameters. Peak inhibition was the most negative response differential among 1 and 3 ms ISIs. Peak facilitation was the maximum positive response differential among 12 to 21 ms ISIs. Response range was the difference of peak facilitation minus peak inhibition, which was to characterize the maximum range or difference between inhibition and facilitation effect from ppTMS. The ICCs for those parameters were also evaluated.
Results
Individualized Facilitation and Inhibition Profile
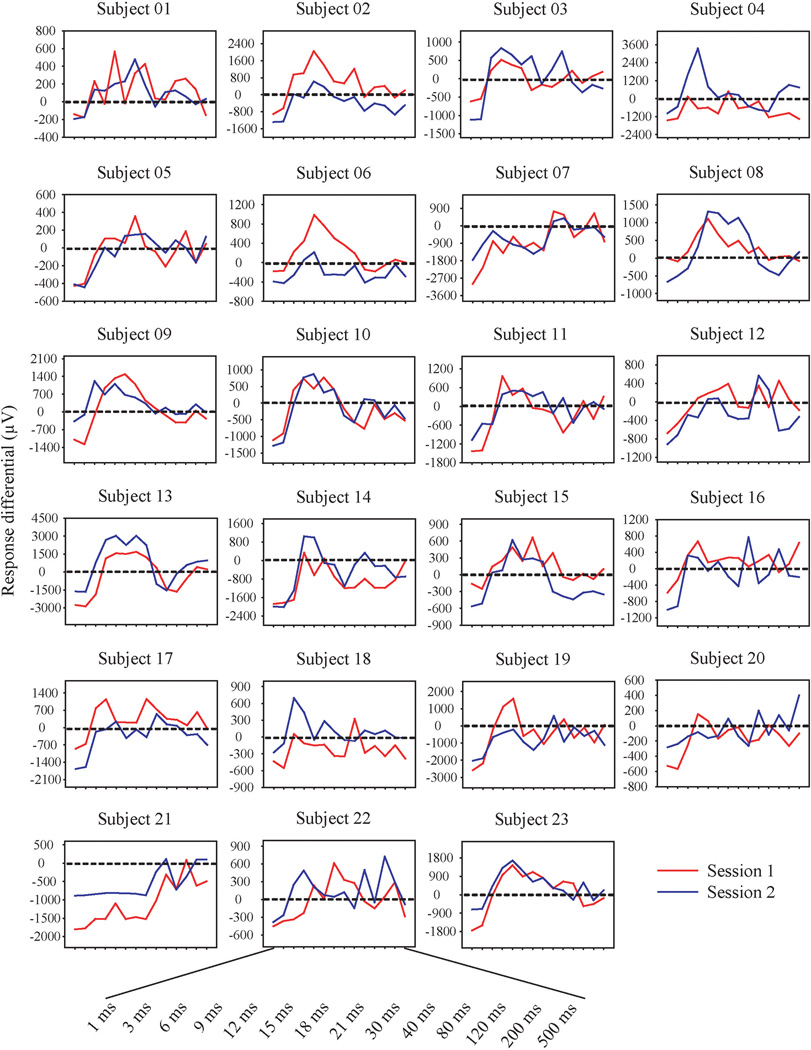
The ppTMS response differentials for each subject across the two sessions were shown in Figure 1 and the grand averages of the response differential of the two sessions across all subjects were shown in Figure 2. By visual inspection of Figure 1, there appeared a large variance in the response profiles across subjects but relatively consistent within-subject profiles across the two sessions in most subjects. To test this observation, we conducted the following analyses.
FIGURE 1.
The ppTMS response differential for each subject across the 14 interstimulus intervals from 1 to 500 ms. Y-axis: Response differential (µV). X-axis; Interstimulus interval. The y-axis has different scales for different subjects because of the wide range of amplitudes across subjects. To assist visual comparisons, the y-axis data were scaled such that the range from the highest peak and the deepest trough within a subject was the same.
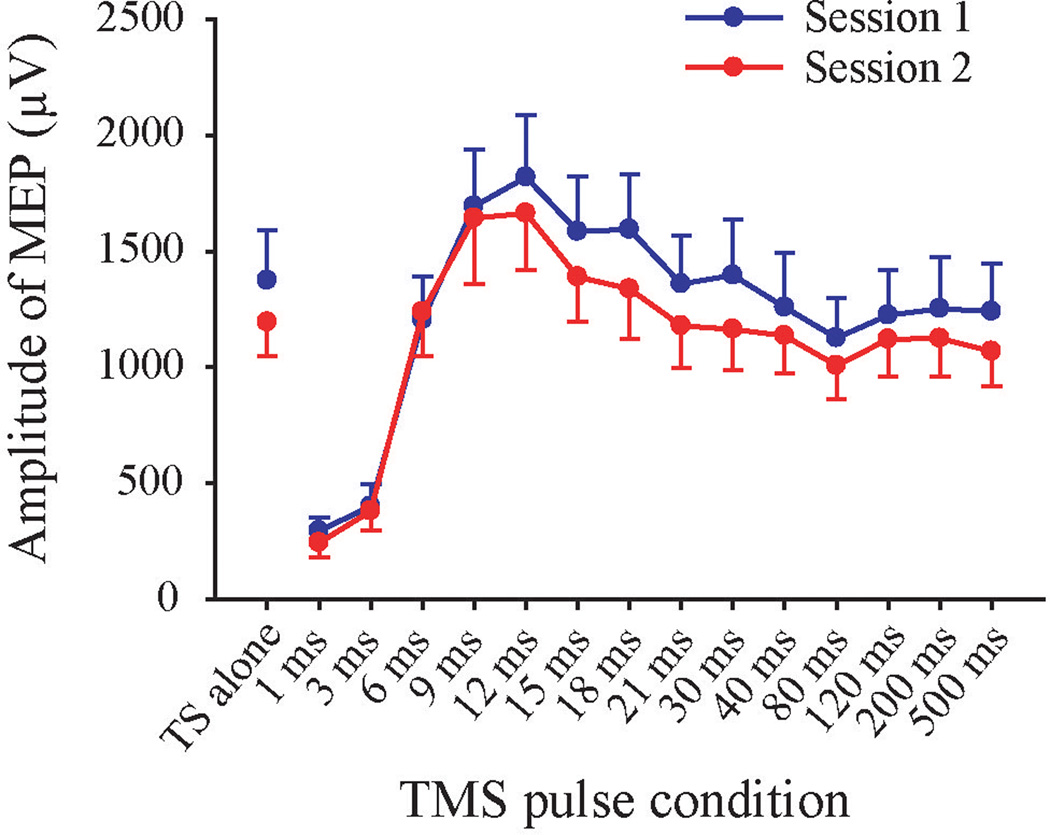
FIGURE 2.
Grand averages of response amplitude at each TMS pulse condition from the two sessions. TS alone: Single test stimulus. On grand average, inhibition was obtained at 1 and 3 ms ISIs since their response amplitudes were smaller than the TS alone; significant facilitation was showed at 9 and 12 ms ISIs. Error bar indicates SEM.
Subjects achieved RMT at 49.0 ± 7.9% (standard deviation, same below) of maximum stimulator output for session 1 and 48.4 ± 7.6% for session 2. No significant inter-session difference was found in RMT (t(22) = 1.26, p = .22). Similarly, the MEP amplitudes of TS alone (at 120% of the RMT simulator strength) did not differ between sessions (t(22) = 1.22, p = .24). MEP amplitudes were 1374 ± 1040 µV for session1 and 1194 ± 705 µV for session 2 (Figure 1). The differences of stimulator output at RMT between session 1 and 2 (intensity difference at RMT) were obtained. The correlations of intensity difference at RMT with differences of amplitude between session 1 and 2 for TS alone and each ISI were not significant (the correlation coefficients were ranged from −0.12 to 0.28 and p values were ranged from .19 to .95). Six subjects were re-tested at both 120% (2138 ± 812 µV) and 150% RMT (5174 ± 2328 µV) (paired t-test: t(5) = −3.85, p = .012) which suggests, on average, participants with 120% RMT stimulation did not reach their physiological ceiling for MEP responses.
For response differential, Mauchly’s test indicated that the assumption of sphericity had been violated for ISIs (χ2 (90) = 304, p < .001) and for session × ISIs (χ2 (90) = 174, p < .001), therefore degrees of freedom were corrected using Greenhouse-Geisser corrections. Repeated measure ANOVA showed that there was no interaction between sessions and ISIs (F(5.34, 117.4) = .54, p = .75). The main effect of session was not significant (F(1, 22) = .33, p = .57), suggesting no systematic difference between the two sessions. A significant main effect for ISI was found (F(2.87, 117.4) = 17.86, p < .0001). Post-hoc tests showed that ppTMS at 1 and 3 ms ISI exhibited inhibition compared with the control single pulse (for 1-ms ISI, t(22) = −7.23, p < .0001 corrected; for 3-ms ISI, t(22) = −7.19, p < .0001 corrected). A significant inhibition was also found for 80-ms ISI (t(22) = −2.96, p = .03 corrected) which might partially reflect the long-interval intracortical inhibition (LICI), although LICI is usually induced by two suprathreshold pulses (Valls-Sole, Pascual-Leone, Wassermann, & Hallett, 1992). Significant facilitations were found at 9 and 12 ms ISIs compared with the control single pulse (for 9-ms ISI, t(22) = 2.78, p = .03 corrected; for 12-ms ISI, t(22) = 3.03, p = .03 corrected). At the group level, other ISIs did not show significant inhibition or facilitation (Figure 2), which is likely the reason that 9 and 12 ms are the commonly recognized ISIs for ICF.
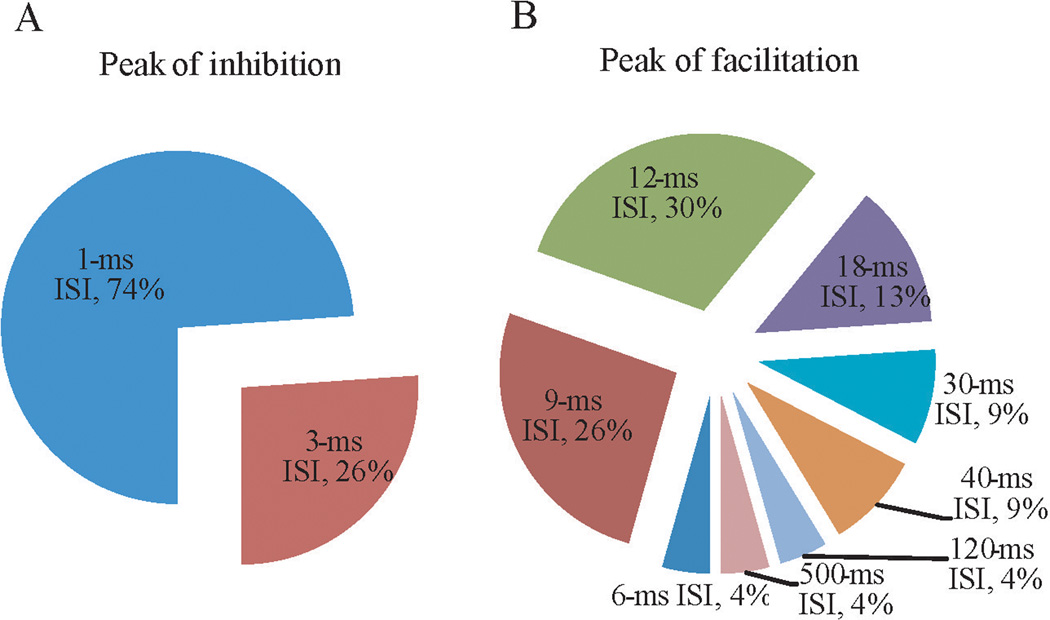
However, statistics that defined inhibition and facilitation for the group may not have sufficiently characterized the individual response patterns: many individuals had clear facilitative effects in other ppTMS ISIs (Figure 1). This is further illustrated by examining the distribution of ISIs with maximum facilitation. For example, only up to 30% of subjects showed maximum facilitation for a single ISI at 12 ms (Figure 3). Two thirds of subjects showed facilitation in other ISIs. The ISIs of maximum facilitation ranged from 6 to 500 ms from individual to individual (Figure 3B). In comparison, the ISIs for maximum inhibition was tight (all at 1 or 3 ms ISI).
FIGURE 3.
Peak-inhibition and peak-facilitation across interstimulus intervals (ISIs). The peak-inhibition (A) occurred only at 1 or 3 ms ISIs. The peak-facilitations (B) were observed at a wide range of ISIs, but mainly at 9, 12 and 18 ms ISIs.
Individualized Facilitation and Inhibition Profile is Reliable
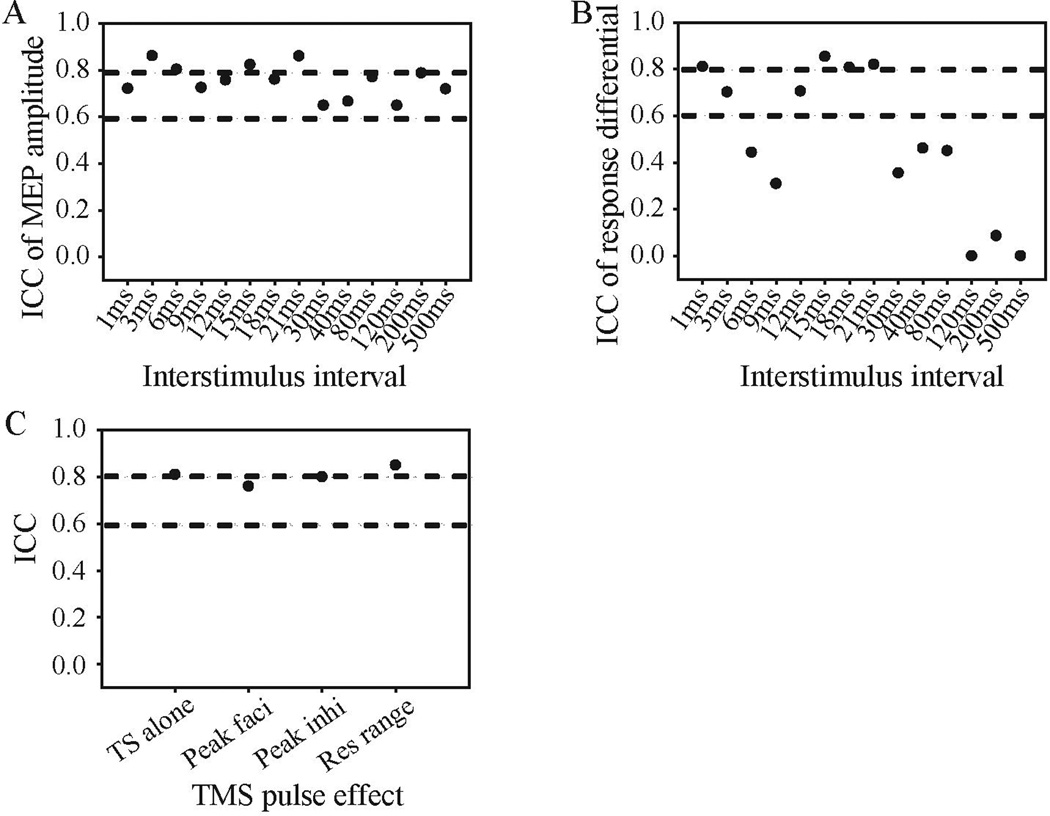
Although the ISI to achieve maximal facilitation varied across subjects, it appeared reliable within subjects. No significant difference between sessions was observed in both raw MEP amplitude and response differential (all ps > 0.05). Good test-retest reliability was obtained for MEP amplitudes of TS alone (ICC = 0.81, 95% confidence interval (CI): 0.56 – 0.92). The ICCs for raw MEP at each ISI were all above 0.6 and at moderate-to-good level (see Figure 4A). For response differential measure, subjects showed acceptable reliability mainly at ISIs that showed inhibitory effects (1 and 3 ms) and facilitatory effects (12 to 21 ms). Specifically, the ICCs for 1 and 3 ms ISIs were 0.81 (95% CI: 0.55 – 0.92) and 0.70 (95% CI: 0.29 – 0.87), respectively. The ICCs for 12 to 21 ms ISIs were ranged from 0.71 to 0.85. On the contrary, poor test-retest reliability was observed for 6 to 9 and then 30 to 500 ms ISIs (Figure 4B). Within the ranges of ISI that showed acceptable reliability, we further sought to define the individualized maximum inhibition and facilitation without the requirement of the same ISI across subjects. In so doing, we found that peak inhibition (the minimum response differential among 1 and 3 ms ISIs; ICC = 0.80, 95% CI: 0.53 – 0.92) and peak facilitation (the maximum response differential among 12 to 21 ms ISIs; ICC = 0.76, 95% CI: 0.44 – 0.90), and response range (peak facilitation – peak inhibition; ICC = 0.85, 95% CI: 0.64 – 0.94) had robust test-retest reliability (Figure 4C). Weighted kappa was used to assess the reliability of ISIs of the peaks, which are treated as ordinal variables, between two sessions (Sim & Wright, 2013). For ISIs of peak-inhibition, the weighted kappa was 0.92. For ISIs of peak-facilitation, the weighted kappa was 0.56. Therefore, almost perfect agreement was observed for peak-inhibition ISIs and moderate agreement was obtained for peak-facilitation ISIs cross sessions (Sim & Wright, 2013).
FIGURE 4.
Intraclass correlation coefficients (ICCs) of ppTMS effects. ICC of ppTMS effects was shown in raw MEP amplitudes (A) and response differential (B). Panel C shows the ICCs for single test stimulus (TS alone), peak facilitation (peak faci), peak inhibition (peak inhi) and response range (res range). All negative ICCs were set to zero for presentation purpose. The lower and upper dash lines indicate ICC of 0.6 and 0.8, respectively, indicating thresholds for acceptable vs. good reliability, respectively.
The ICC pattern described above was replicated in both male and female groups; the acceptable reliability of response differential for males and females was exhibited mainly at SICI ISIs (1 and 3 ms) and ICF ISIs (12 to 21 ms). For females, acceptable ICCs were found at 1 to 3 ms ISIs (ICCs ranged from 0.71 to 0.86) and 15 to 21 ms ISIs (ICCs ranged from 0.75 to 0.77). For males, acceptable ICCs were observed at 1 to 3 ms ISIs (ICCs ranged from 0.76 to 0.80) and 12 to 21 ms ISIs (ICCs ranged from 0.77 to 0.92). Furthermore, both gender groups showed moderate-to-good ICCs at peak facilitation, peak inhibition and response range (for female, ranged from 0.62 to 0.85; for male, ranged from 0.80 to 0.88).
Discussion
In the present study we systematically evaluated the individualized pattern of paired-pulse TMS inhibition and facilitation. Consistent with previous studies, averaged ISI windows of the paired-pulse inhibition and facilitation effects were in the 1 – 3 ms and 9 – 12 ms range, respectively. However, our study highlighted the phenomenon of large variability across individuals in the ISI windows in which inhibition and facilitation occur. Although highly variable across subjects in the ISI in which the inhibition and facilitation were observed, the pattern was remarkably reliable.
The raw MEP amplitudes showed acceptable reliability across all TMS conditions in TS alone and in ppTMS at all ISIs. On the other hand, the ppTMS effects measured as response differentials exhibited moderate-to-good reliability only at typical SICI in 1 and 3 ms and ICF ISIs in 12 to 21 ms, but not other ISIs 6 to 9 ms and 30 to 500 ms. The reason why some ISIs showed larger variability across sessions is unclear. It may be due in part to reduced signal fidelity in these ISIs, which had similar amplitudes compared with the single test pulse MEP and thus reduced signal-to-noise ratio in these measures (see Figure 2 for their respective magnitudes in relationship to the single test pulse MEP amplitude). Other possible causes can be delineated. Pharmacological studies suggested SICI is mediated through a GABAA receptor-dependent pathway as it is significantly increased by the administration of GABAA receptor agonist such as benzodiazepines (Di Lazzaro et al., 2000; Hanajima et al., 1998; Ilic et al., 2002; Ziemann, Lonnecker, Steinhoff, & Paulus, 1996a, 1996b). In comparison, complex mechanisms may underlie ICF, as ICF is affected by both NMDA (Liepert, Schwenkreis, Tegenthoff, & Malin, 1997; Ziemann, Rothwell, & Ridding, 1996) and GABAA receptor agents (Ziemann, 2004; Ziemann, Lonnecker, Steinhoff, & Paulus, 1996a). Therefore, another possible, yet speculative explanation for the higher variability of ICF measures as compared to the SICI measures could be related to the more complex mechanisms involved in generating ICF.
Overall, by means of selecting proper parameters and maintaining precise procedures, we found acceptable test-retest reliability for both facilitation and inhibition effects. Our findings also demonstrated that individualized ppTMS facilitation and inhibition may yield important trait-like information. Specifically, the peak facilitation, peak inhibition and maximum inhibition/facilitation range (response range) showed reasonable test-retest reliability, suggesting that these derived individualized measures may index certain trait-like cortical functions.
Several studies examined the reproducibility of SICI and ICF using paired-pulse paradigms. In one study, ppTMS effects at 1.5 to 20 ms ISIs were tested and no significant inter-session difference was found, although ICF showed smaller inter-session difference than SICI (Boroojerdi et al., 2000). In contrast, Maeda and colleagues found SICI, but not ICF, to be reproducible (Maeda et al., 2002). However, in their study, different ISIs were grouped together to indicate SICI (grouped from 1.5 to 3 ms ISIs) and ICF (grouped from 8 to 20 ms ISIs). By using two static ISIs (3 and 15 ms ISIs) to examine SICI and ICF, Orth and colleagures found considerable variation between sessions although the differences were not statistically significant (Orth et al., 2003). The reliability of ppTMS effects was not evaluated by intraclass correlation coefficient (ICC) until recently (Fleming et al., 2012) and Fleming and colleagues found good reliability for SICI with 2.5 ms ISI, but not for ICF with 12.5 ms ISI. Therefore, either insufficient or more variable reliability were reported in previous studies. By more stringent coil positioning, randomization of stimulus sequences, through assessment on a wide range of ppTMS effects at various ISIs, we found acceptable reliability at both SICI (1 and 3 ms ISIs) and ICF ISIs (12 to 21 ms ISIs).
Another factor that may account for the different findings is the dependent variables used. In our study, we found that raw MEP amplitudes were highly reliable across TMS conditions. Therefore, ppTMS effects were calculated by subtracting MEP of TS alone from MEP of each ISI, i.e., the response differential. Most previous studies used a ratio of MEP of each ppTMS ISI to MEP of single pulse TMS (i.e., relative MEP or normalized MEP). Our results suggested that ppTMS effects can be more reliable when measured as response differentials.
The selection of the intensities of conditioning and test pulse for SICI and ICF may also be important for improving the reliability of SICI/ICF effects: 80% of RMT for conditioning TMS pulse was used in many other studies as well (Kujirai et al., 1993; Maeda et al., 2002; Orth et al., 2003) and was proven to be one of the best intensities to induce ICF (Kossev, Siggelkow, Dengler, & Rollnik, 2003). The intensity of the test TMS pulse here was set to 120% of RMT which was suggested to be the best suprathreshold intensity to elicit SICI. Higher or lower (< 110% RMT) intensity resulted in reduction of SICI (Garry & Thomson, 2009). Relatively small ICF in our study is unlikely due to a physiological ceiling of the maximum MEP possible with TMS, because when a higher intensity (i.e., 150 % RMT) was tested in a few subjects, we found that subjects could reliably show larger MEPs. Furthermore, the coil positioning in our study was kept relatively constant using a frameless navigation system (Brainsight). The precise positioning of the coil may have also contributed to the reduction of MEP variability.
Based on our findings, the peak inhibition and peak facilitation were reliable within subjects but can be variable between subjects. An important implication of the present work is that future research using a paired-pulse paradigm should consider pre-testing characterizations of participants’ ICI and ICF profiles so that the specific peaks can be targeted. The mechanisms leading to the individualized peaks are unclear. It is possible that individual differences in the peaks are controlled by activation of different circuits, different receptors types, or different neural chemistry and receptor levels within the same circuits. These individually stable measures may also serve as phenotypes for genetic research to identify possible genes associated with these peak inhibition and facilitation. In that sense, this work may aid future research aiming to examine the underlying mechanism of the individual variability in peak ICI and ICF.
There are some limitations of the study. First, the number of repetitions (6 repetitions for each ISI and 12 repetitions for TS alone) is relatively small, although we found acceptable reliability between sessions using these numbers of repetitions. Exploratory analysis using median, outlier removal using two standard deviations, outlier removal based on root mean squared of the amplitudes, etc, revealed similar inter-subject variability results. Secondly, lack of manipulation on the intensity of CS and TS (e.g., using subthreshold-CS and subthreshold-TS pairs, or suprathreshld-CS and suprathreshld-TS pairs) limited our conclusion to subthreshold-CS and suprathreshold-TS ppTMS condition (SICI and ICF). The inter-subject variability and inter-session reliability for long-interval intracortical inhibition (LICI, suprathreshld-CS and suprathreshld-TS pairs) and short-interval intracortical facilitation (SICF, suprathreshld-CS and subthreshold-TS pairs) need further exploration. Third, a low pass filter at 100 Hz for EMG was used here as compared to higher frequency low pass filters more commonly used and recommended. This may introduce some loss in high frequency information and could reduce the amplitude of MEP contributed by MEP above 100 Hz.
The present data showed high inter-subject variability yet reasonable within-subject reliability on intracortical inhibition and facilitation, suggesting that individuals have unique inhibition/facilitation profiles that are relatively stable. Although the functional implications of individualized profiles are currently unknown, the relatively stable profiles that are highly variable across individual may index certain individualized underlying neural inhibition and excitation properties.
Acknowledgements
This research was supported by NIH grants MH085646, DA027680, MH049826, and MH077852.
Footnotes
Financial Disclosures:
All authors report no conflict of interests.
Reference
- Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Changes in cortical excitability differentiate generalized and focal epilepsy. Ann Neurol. 2007;61(4):324–331. doi: 10.1002/ana.21087. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Kopylev L, Battaglia F, Facchini S, Ziemann U, Muellbacher W, Cohen LG. Reproducibility of intracortical inhibition and facilitation using the paired-pulse paradigm. Muscle & Nerve. 2000;23(10):1594–1597. doi: 10.1002/1097-4598(200010)23:10<1594::aid-mus19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol. 1992;85(1):9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- Chu J, Wagle-Shukla A, Gunraj C, Lang AE, Chen R. Impaired presynaptic inhibition in the motor cortex in Parkinson disease. Neurology. 2009;72(9):842–849. doi: 10.1212/01.wnl.0000343881.27524.e8. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cavalli A, Naldi P, Varrasi C, Cantello R. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clin Neurophysiol. 2000;111(4):624–629. doi: 10.1016/s1388-2457(99)00301-6. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Transcranial magnetic stimulation: a new investigational and treatment tool in psychiatry. J Neuropsychiatry Clin Neurosci. 2002;14(4):406–415. doi: 10.1176/jnp.14.4.406. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111(5):794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Du X, Chen L, Zhou K. The role of the left posterior parietal lobule in top-down modulation on space-based attention: a transcranial magnetic stimulation study. Hum Brain Mapp. 2012;33(10):2477–2486. doi: 10.1002/hbm.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143(2):240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Fleming MK, Sorinola IO, Newham DJ, Roberts-Lewis SF, Bergmann JH. The effect of coil type and navigation on the reliability of transcranial magnetic stimulation. IEEE Trans Neural Syst Rehabil Eng. 2012;20(5):617–625. doi: 10.1109/TNSRE.2012.2202692. [DOI] [PubMed] [Google Scholar]
- Garry MI, Thomson RH. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp Brain Res. 2009;193(2):267–274. doi: 10.1007/s00221-008-1620-5. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Ridel KR, Sallee FR, Zhang J, Lipps TD, Wassermann EM. Comparison of the Inhibitory and Excitatory Effects of ADHD Medications Methylphenidate and Atomoxetine on Motor Cortex. Neuropsychopharmacology. 2005;31(2):442–449. doi: 10.1038/sj.npp.1300806. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531(Pt 3):849–859. doi: 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509(Pt 2):607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545(Pt 1):153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112(2):250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Albrecht UV, Caltagirone C, Rothwell JC. Effects of paired pulse TMS of primary somatosensory cortex on perception of a peripheral electrical stimulus. Exp Brain Res. 2006;172(3):416–424. doi: 10.1007/s00221-006-0359-0. [DOI] [PubMed] [Google Scholar]
- Kossev AR, Siggelkow S, Dengler R, Rollnik JD. Intracortical inhibition and facilitation in paired-pulse transcranial magnetic stimulation: effect of conditioning stimulus intensity on sizes and latencies of motor evoked potentials. J Clin Neurophysiol. 2003;20(1):54–58. doi: 10.1097/00004691-200302000-00007. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Bar KJ, Meske U, Weiller C. Motor cortex disinhibition in Alzheimer's disease. Clin Neurophysiol. 2001;112(8):1436–1441. doi: 10.1016/s1388-2457(01)00554-5. [DOI] [PubMed] [Google Scholar]
- Liepert J, Schwenkreis P, Tegenthoff M, Malin JP. The glutamate antagonist riluzole suppresses intracortical facilitation. J Neural Transm. 1997;104(11–12):1207–1214. doi: 10.1007/BF01294721. [DOI] [PubMed] [Google Scholar]
- Liu SK, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The Relationship Between Cortical Inhibition, Antipsychotic Treatment, and the Symptoms of Schizophrenia. Biological psychiatry. 2009;65(6):503–509. doi: 10.1016/j.biopsych.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113(3):376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Pascual-Leone A. Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. Br J Psychiatry. 2000;177:169–173. doi: 10.1192/bjp.177.2.169. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70(1):19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498(Pt 3):817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazarán J, Prieto J, Cruz I, Esteban A. Cortical excitability in very mild Alzheimer’s disease: a long-term follow-up study. Journal of Neurology. 2010;257(12):2078–2085. doi: 10.1007/s00415-010-5663-8. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Caltagirone C, Filippi MM, Traversa R, Cicinelli P, Pasqualetti P, Rossini PM. Paired transcranial magnetic stimulation protocols reveal a pattern of inhibition and facilitation in the human parietal cortex. J Physiol. 2000a;529(Pt 2):461–468. doi: 10.1111/j.1469-7793.2000.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Filippi MM, Traversa R, Cicinelli P, Palmieri MG, Pasqualetti P, Caltagirone C. Time-dependent activation of parieto-frontal networks for directing attention to tactile space. A study with paired transcranial magnetic stimulation pulses in right-brain-damaged patients with extinction. Brain. 2000b;123(Pt 9):1939–1947. doi: 10.1093/brain/123.9.1939. [DOI] [PubMed] [Google Scholar]
- Orth M, Amann B, Robertson MM, Rothwell JC. Excitability of motor cortex inhibitory circuits in Tourette syndrome before and after single dose nicotine. Brain. 2005;128(6):1292–1300. doi: 10.1093/brain/awh473. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114(12):2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Orth M, Münchau A, Rothwell JC. Corticospinal System Excitability at Rest Is Associated with Tic Severity in Tourette Syndrome. Biological psychiatry. 2008;64(3):248–251. doi: 10.1016/j.biopsych.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Upper Saddle River, NJ: Pearson & Prentice Hall; 2009. [Google Scholar]
- Rankin G, Stokes M. Reliability of assessment tools in rehabilitation: an illustration of appropriate statistical analyses. Clin Rehabil. 1998;12(3):187–199. doi: 10.1191/026921598672178340. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Ann Neurol. 1995;37(2):181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551(Pt 2):649–660. doi: 10.1113/jphysiol.2003.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151(3):330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68(7):484–488. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- Russmann H, Lamy JC, Shamim EA, Meunier S, Hallett M. Associative plasticity in intracortical inhibitory circuits in human motor cortex. Clin Neurophysiol. 2009;120(6):1204–1212. doi: 10.1016/j.clinph.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisanen L, Julkunen P, Niskanen E, Hukkanen T, Mervaala E, Karhu J, Kononen M. Short- and intermediate-interval cortical inhibition and facilitation assessed by navigated transcranial magnetic stimulation. J Neurosci Methods. 2011;195(2):241–248. doi: 10.1016/j.jneumeth.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sim J, Wright CC. The kappa statistic in reliability studies: use interpretation, and sample size requirements. Phys Ther. 2005;85(3):257–268. [PubMed] [Google Scholar]
- Sommer M, Classen J, Cohen L, GHallett M. Time course of determination of movement direction in the reaction time task in humans. J Neurophysiol. 2001;86(3):1195–1201. doi: 10.1152/jn.2001.86.3.1195. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroenceph Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Udupa K, Ni Z, Gunraj C, Chen R. Effect of long interval interhemispheric inhibition on intracortical inhibitory and facilitatory circuits. J Physiol. 2010;588(Pt 14):2633–2641. doi: 10.1113/jphysiol.2010.189548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85(6):355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Vucic S, Cheah BC, Krishnan AV, Burke D, Kiernan MC. The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Res. 2009;1273:39–47. doi: 10.1016/j.brainres.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113(7):1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93(2):138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Schneider-Axmann T, Retz W, Rosler M, Kadovic D, Falkai P, Schneider M. Motor circuit abnormalities in first-episode schizophrenia assessed with transcranial magnetic stimulation. Pharmacopsychiatry. 2009;42(5):194–201. doi: 10.1055/s-0029-1224137. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Schneider M, Kadovic D, Schneider-Axmann T, Ecker UK, Retz W, Rösler M, Falkai P. Reduced cortical inhibition in first-episode schizophrenia. Schizophr Res. 2008;105(1–3):252–261. doi: 10.1016/j.schres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Hasan A, Malchow B, Wolff-Menzler C, Guse B, Lang N, Schneider-Axmann T, Ecker UK, Falkai P. Increased cortical inhibition deficits in first-episode schizophrenia with comorbid cannabis abuse. Psychopharmacology. 2010;208(3):353–363. doi: 10.1007/s00213-009-1736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115(8):1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109(1):127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996b;40(3):367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;51:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]