Table 1.
Oxidative amination optimization.[a]
| Entry | CuX2·ligand | Base | Solvent |
T [°C] |
Yield [%][b] |
|---|---|---|---|---|---|
| 1 | Cu(OTf)2·2,2’-bipyridine | K2CO3 | CF3Ph | 120 | 65 |
| 2 | Cu(eh)2 | K2CO3 | CF3Ph | 120 | 70 |
| 3[c] | Cu(eh)2 | K2CO3 | CF3Ph | 120 | 50 |
| 4 | Cu(OTf)2·7 | k2co3 | CF3Ph | 120 | 90[d] |
| 5 | Cu(OTf)2·7 | – | CH3Ph | 120 | 90[d] |
| 6[e] | Cu(OTf)2·7 | – | CH3Ph | 120 | 80 |
| 7[f] | Cu(OTf)2·7 | – | CH3Ph | 120 | 25 |
| 8 | Cu(OTf)2·7 | – | DCE | 105 | 65 |
| 9 | Cu(OTf)2·7 | k2co3 | DCE | 105 | 65 |
| 10 | Cu(OTf)2·7 | 8 | DCE | 105 | 85[d] |
| 11[g] | – | – | CH3Ph | 120 | 20 |
| 12[h] | – | – | CH3Ph | 120 | n.r. |
| 13[i] | Cu(OTf)2·7 | – | CH3Ph | 120 | 60 |
| 14[j] | Cu(OTf)2·7 | – | CH3Ph | 120 | 70 |
| 15[k] | Cu(OTf)2·7 | – | CH3Ph | 120 | 67 |
| 16 | Cu(0Tf)2·1,10-phenanthro- line |
– | CH3Ph | 120 | 85 |
| 17[l] | Cu(OTf)2·7 | 8 | DCE | 105 | 15 |
| 18[m] | Cu(OTf)2·7 | – | CH3Ph | 120 | 50 |
| 19[n] | Cu(OTf)2·7 | – | CH3Ph | 120 | trace |
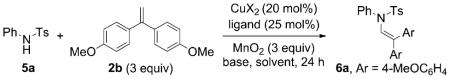
Conditions: Cu(X)2 (0.04 mmol) and ligand (0.05 mmol) in solvent (1 mL) were heated for 2 h at 60 °C under Ar. Sulfonamide 5a (0.20 mmol), MnO2 (0.60 mmol), solvent (1 mL), base (0.20 mmol), and flame-dried 4 Å molecular sieves (40 mg) were added. The solution was heated for 24 h in a sealed tube, then filtered through SiO2 and concentrated.
onversion [%] based on crude NMR analysis.
Used copper(II) 2-ethylhexanoate [Cu(eh)2] (3 equiv) and no oxidant.
Isolated yield following flash chromatography on silica gel.
Run with 15 mol% Cu(OTf)2 and 18mol% 7.
Run with 50mol% Cu(OTf)2·7 in the absence of MnO2.
Run in then absence of a copper salt.
Run in the absence of MnO2 and CuX2.
Run with 2b (1 equiv).
Run with 2b (2 equiv).
Run with 2b (1 equiv), Cu(OTf)2 (30 mol%), and 7 (38 mol%). N.r. = no reaction.
O2 (1 atm, balloon) was used instead of MnO2.
Run with MnO2 (60 mol%) under O2 (1 atm, balloon).
Run with 60 mol% MnO2.