Table 3.
Synthesis of indoles and larger ring enamides.[a]
| Entry | Substrate | Product | Yield [%][b] |
|---|---|---|---|
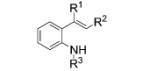
|
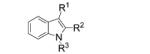
|
||
| 1 | 12a, R1, R2 = H, R3 = Ts | 13 a, R1, R2 = H, R3 = Ts | 66 |
| 2[c] |
12b, R1 = Me, R2 = H, R3 = Ts |
13 b, R1 = Me, R2 = H, R3 = Ts |
90 |
| 3[c] |
12 c, R1 = Ph, R2 = H, R3 = Ts |
13 c, R1 = Ph, R2 = H, R3 = Ts |
97 |
| 4[d] | 12 c | 13 c | 70 |
| 5 |
12d, R1 = H, R2 = Ph, R3 = Ts |
13 d, R1 = H, R2 = Ph, R3 = Ts |
41 |
| 6[c] |
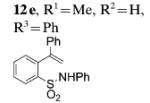
|
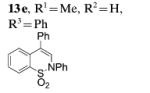
|
95 |
| 7[c] |
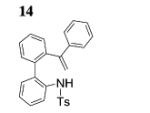
|
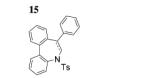
|
82 |
| 8 | 16 | 17 (unsaturated)/ | 94 |
| 18 (saturated) | (5:1) | ||
| 9[e] |
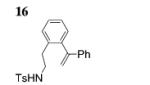
|
17 (exclusively) | 90 |
| 10 | 19 | n.r. |
Same conditions as in Table 1 for entry 5 were used, except no external alkene 2 was added.
Isolated yield.
Reaction run with 5 mol % Cu(OTf)2·7.
Reaction run in the absence of copper.
Reaction run in CF3Ph instead of CH3Ph.
