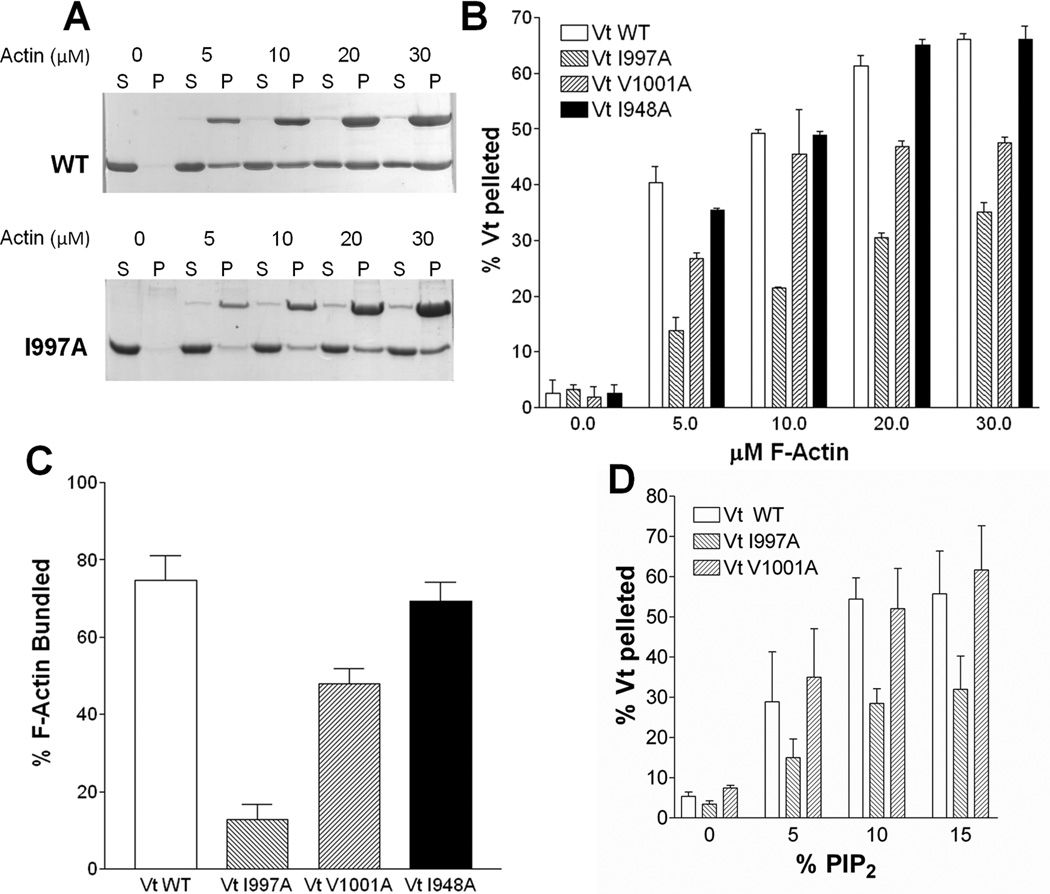
Figure 1. VtI997A and VtV1001A are deficient in F-actin binding and bundling yet retain association with PIP2.
(A) SDS-polyacrylamide gel electrophoresis of supernatant (S) and pellet (P) fractions after co-sedimentation of Vt with F-actin. Actin concentrations and Vt variants are noted. (B) Quantification of F-actin co-sedimentation assays identifies Vt variants in H4 deficient in F-actin binding. (C) Vt variants deficient in binding to F-actin are also defective in F-actin bundling. (D) VtV1001A, while deficient in actin binding, retains PIP2 binding comparable to VtWT. VtI997A is impaired in PIP2 binding. Error bars are standard deviation, n= 3. See also Figure S1.