Figure. Role of IRAK1 complex in sustaining MDS- and AML-propagating cells.
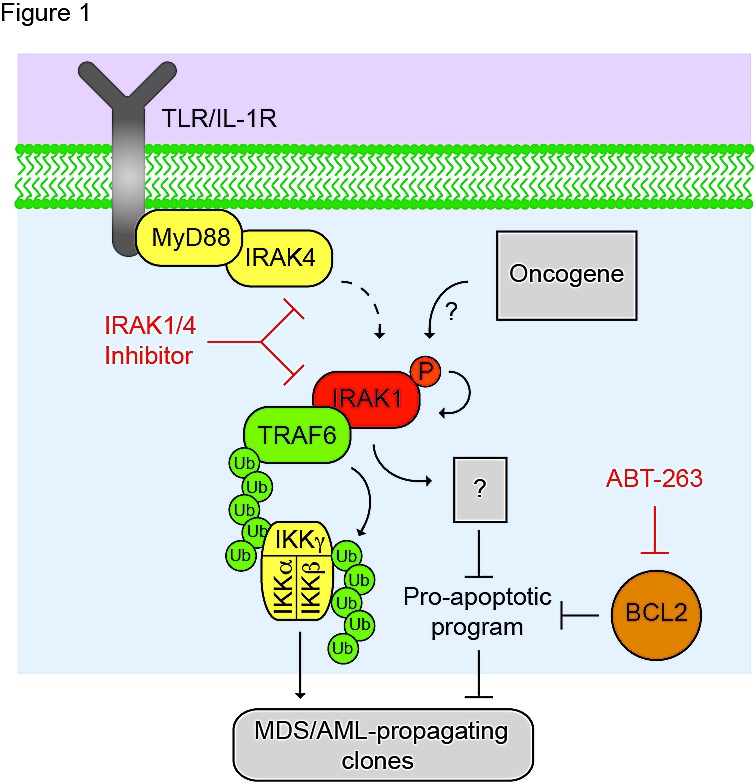
IRAK1 is a kinase downstream from Toll-like receptor (TLR) and Interleukin-1 Receptor (IL1R). Following receptor activation under normal conditions, IRAK1 becomes phosphorylated which then leads to recruitment of TRAF6. In the context of MDS or AML, IRAK1 is activated (phosphorylated) in the absence of TLR activation, either through cross-phosphorylation or by an oncogenic kinase. Activated TRAF6 becomes autoubiquitinated by forming lysine (K)-63 linkages, which serve as a scaffold for the NF-κB kinase complex (IKKα/β/γ). TRAF6 subsequently ubiquitinates the NF-κB complex resulting in NF-κB activation. IRAK1, and likely TRAF6, have the potential to regulate other pathways relevant to sustaining the MDS/AML clones. IRAK1/4-Inh selectively targets IRAK1 and IRAK4, and inhibits TRAF6-mediated NF-κB activation. IRAK-Inh is not efficient at inhibiting anti-apoptotic family members (e.g., BCL2). To counteract this phenomenon, co-treatment with ABT-263 synergistically targets the MDS/AML-propagating cells.
