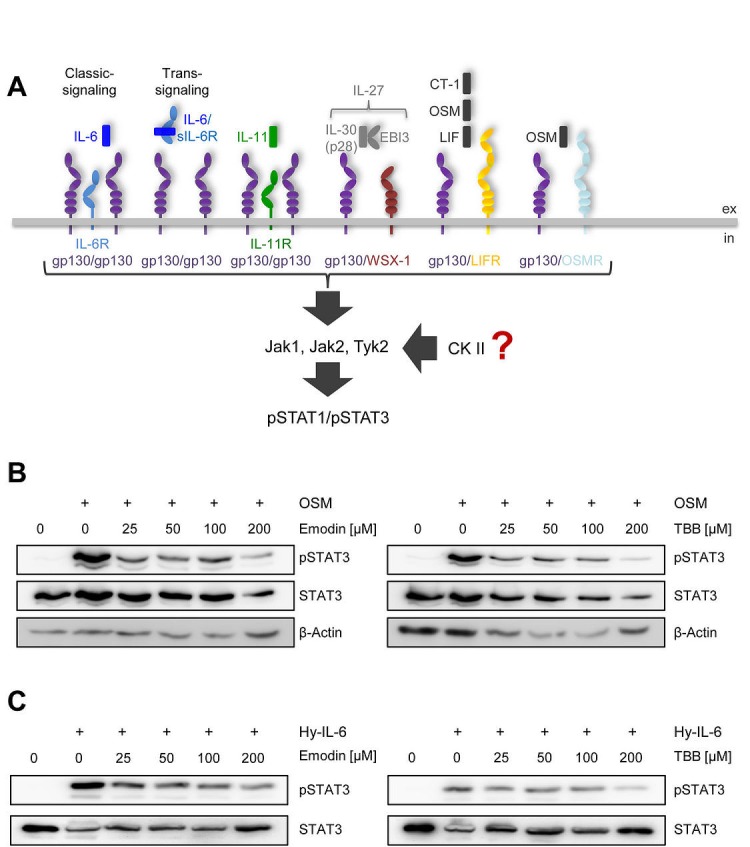
Fig 1. CK2 is involved in STAT3 activation by OSM and Hyper-IL-6.
(A) Schematic overview of the members of the IL-6 cytokine family and their receptors investigated in this study. IL-6 can activate a homodimer of glycoprotein 130 (gp130) either via the membrane-bound IL-6R (classic signaling) or via the soluble IL-6R (trans-signaling), whereas IL-11 acts only via a membrane-bound IL-11R. IL-27 (p28/IL-30 and EBI3) engages a gp130/WSX-1 heterodimer. The three members CT-1, OSM and LIF share a heterodimer of gp130/LIFR as signal transduction complex, while OSM can in addition also activate gp130 in combination with OSMR. IL-6 family cytokines activate the three kinases Jak1, Jak2 and Tyk2, which in turn phosphorylate STAT1 and STAT3. The influence of CK2 on this signaling pathway is investigated in the current study. (B) HepG2 cells were treated with different concentrations of the two CK2-inhibitors Emodin and TBB for 90 min. Cells were afterwards stimulated with 10 ng/ml OSM for 15 min. Phosphorylation of STAT3 was assessed by Western blotting. (C) HepG2 cells were treated as described under panel B, but were stimulated with 10 ng/ml Hyper-IL-6. Phosphorylation of STAT3 was assessed by Western blotting. One representative experiment of two performed is shown.