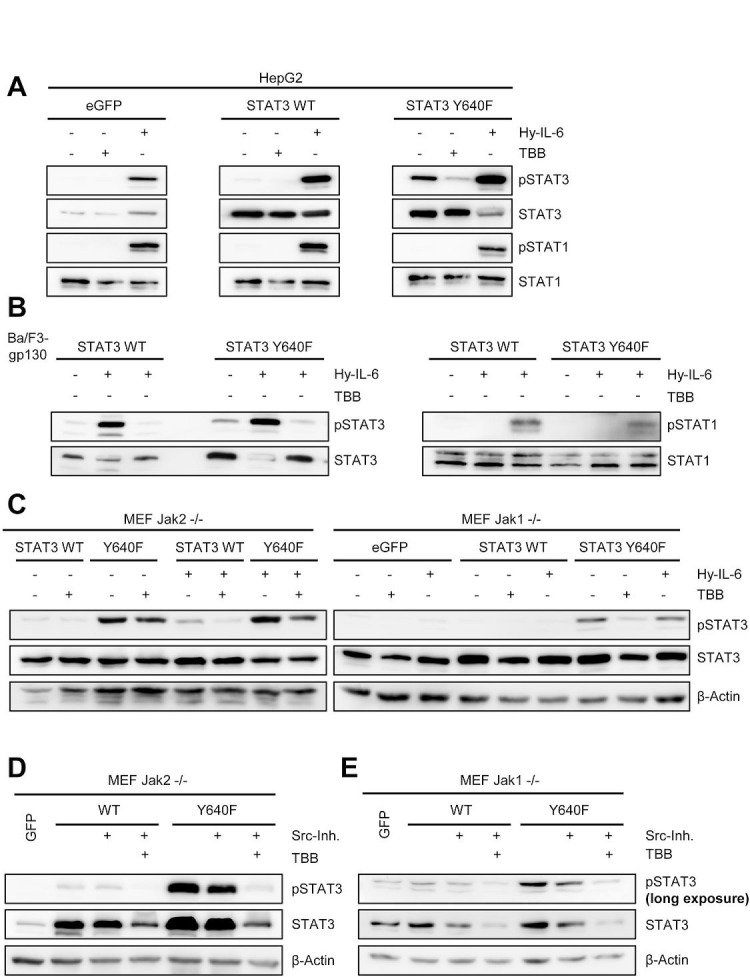
Fig 8. Activity of CK2 is required for phosphorylation of a constitutively active STAT3 mutant.
(A) HepG2 cells were transiently transfected with eGFP, STAT3wt or STAT3Y640F. Two days later, cells were serum-starved for 5h and stimulated with either 10 ng/ml Hyper-IL-6 or were pre-treated with 100 μM TBB for 90min where indicated. (B) Ba/F3-gp130 cells stably transduced with STAT3wt or STAT3Y640F were serum-starved for 3 h and stimulated with either 10 ng/ml Hyper-IL-6 or were pre-treated with 100 μM TBB for 90 min where indicated. Phosphorylation of STAT1 and STAT3 was determined by Western blotting, and STAT1/3 served as internal loading control. (C) Jak2−/− or Jak1−/− MEFs were transiently transfected with a plasmid coding for eGFP, STAT3wt or STAT3Y640F. Two days later, cells were serum-starved for 5 h and stimulated with either 10 ng/ml Hyper-IL-6 or were pre-treated with 75 μM TBB for 90 min where indicated. Phosphorylation of STAT3 was determined by Western blotting, and STAT3/β-actin served as internal loading control. (D, E) Jak2−/− or Jak1−/− MEFs were transiently transfected with a plasmid coding for eGFP, STAT3wt or STAT3Y640F. Two days later, cells were serum-starved for 5 h and treated with Src-inhibitor alone or Src-inhibitor in combination with TBB for 90 min. Phosphorylation of STAT3 was determined by Western blotting, and STAT3/β-actin served as internal loading control. Western Blots show one representative experiment of three performed.