Acute myocardial infarction (MI) triggers healing and compensatory responses that serve to restrain damage and maintain cardiac output in the face of myocyte death. These encompass both local events (e.g., inflammatory cell infiltration, efferocytosis, myofibroblast transformation, and extracellular matrix turnover) and global events (e.g., neurohormonal activation and augmented wall stress) that ultimately result in the generation of a stable infarct scar capable of withstanding distending forces together with variable degrees of left ventricular (LV) enlargement.1–3 The size of the initial infarct and the adequacy of the ensuing wound healing response are both key determinants of the attendant LV dilatation and dysfunction that occurs after MI, and the subsequent progression to late remodeling and heart failure. In humans, the degree of LV dilatation following MI is the primary determinant of late mortality.4, 5
Immune cells and wound healing post-MI
Immune cells are critical for local infarct zone remodeling.3, 6–15 Both innate and adaptive immune cell types dynamically infiltrate into the infarct, with the highest activity 1 w post-MI, followed by a subsequent decline.10 Following the early appearance of neutrophils, monocytes/macrophages are the predominant cells and display phasic functional heterogeneity that serve to guide proper wound healing. In a seminal report, Swirski, Nahrendorf and co-workers demonstrated that monocytes are rapidly recruited from a splenic reservoir to the infarcted heart where they accumulate and participate in tissue repair.7 Subsequent studies from these and other investigators8–10 suggested that monocytes/macrophages infiltrate the infarcted heart in two phases - an early (~3–4 d) peak of pro-inflammatory (Ly-6Chi) monocytes and M1 macrophages that promote tissue digestion, followed by a late (~7–8 d) peak of reparative Ly-6Clow monocytes and M2 macrophages that resolve inflammation and promote scar formation and neovascularization - and that proper M1 to M2 macrophage transition enhances tissue repair.11, 12 In addition to macrophages, dendritic cells,13 CD4+ T-cells,14 and B-cells15 have also been shown to regulate wound healing post-MI, in part by impacting monocyte recruitment kinetics and/or the pro- vs. anti-inflammatory macrophage balance in healing myocardium.
Critical re-evaluation of the biphasic monocyte/macrophage infiltrative response
It was originally proposed that this biphasic tissue macrophage response after MI resulted from the sequential recruitment of Ly6Chi and Ly6Clow monocytes from the circulation into the heart;8, 9 however, the aforementioned studies did not employ flow cytometry strategies that were sufficiently stringent to discriminate between Ly6C expressing monocytes vis-à-vis macrophages. Moreover, the sequential monocyte recruitment model was at odds with recent studies demonstrating that Ly6Clow monocytes do not contribute to the pool of macrophages generated from extravasated monocytes during inflammation, but rather provide vascular surveillance and orchestrate the killing of damaged endothelial cells.16, 17 This paradigm also contrasted with studies of skeletal muscle18 and hepatic injury,19 which indicated that reparative Ly6Clow macrophages derive from local phenotypic switching of pro-inflammatory Ly6Chi monocytes in response to environmental cues. These observations suggested a need for reappraisal of this schema of macrophage infiltration in the post-MI heart.
Accordingly, in this issue of Circulation Research, using an elegant study design, Hilgendorf et al.20 advance their previous studies and provide important and definitive insights into both the source and role of pro-inflammatory and reparative macrophages in the infarcted heart. Using highly stringent flow cytometry approaches, they evaluated post-MI monocyte and macrophage infiltration in chimeric mice deficient for the orphan nuclear hormone receptor Nr4a1 in hematopoietic cells (Nr4a1−/− mice); this receptor is essential for the development and survival of Ly6Clow monocytes.21 Notably, chimeric Nr4a1−/− mice still exhibited the biphasic monocyte/macrophage response, despite the demonstrated absence of circulating Ly6Clow monocytes. When taken together with Ly6Chi and Ly6Clow monocyte fate-mapping experiments, the data indicated that both phases derive from circulating Ly6Chi monocytes - an initial pro-inflammatory Ly6Chi monocyte (and macrophage) dominant phase and a delayed Ly6Chi monocyte-derived reparative Ly6Clow macrophage phase.
The microenvironment in the infarcted heart supported phenotypic switching to reparative macrophages exhibiting augmented Nr4a1 expression in wild-type mice, as well as relatively robust local macrophage proliferation in both chimeric Nr4a1−/− and wild-type mice. Loss of Nr4a1 in hematopoietic cells resulted in: 1) enhanced Ly6Chi monocyte Ccr2 expression and a significant Ccr2-dependent augmentation of mobilization and cardiac infiltration of Ly6Chi monocytes; 2) exaggerated pro-inflammatory features in cardiac macrophages (presumably both Ly6Chi and Ly6Clow macrophages); and 3) exacerbation of post-MI LV remodeling with larger and less compact scars, and greater LV dilatation and dysfunction. Hence, in the infarcted heart, Ly6Chi monocytes exhibit temporal singularity of sorts, a state from which they may be channeled into becoming either pro-inflammatory or anti-inflammatory infiltrating cells depending on local cues. Nr4a1 plays an important role in this process, serving as a critical driver of anti-inflammatory and reparative polarity in macrophages. These findings establish that the cellular derivation of the biphasic macrophage response post-MI is exclusively from pro-inflammatory monocytes, with a corollary that macrophage Nr4a1 may be a potential therapeutic target in the quest to limit adverse remodeling.
The changing paradigm for inflammatory cells in LV remodeling: implications for future research
These pivotal results raise several important translational and conceptual considerations (Figure). Current therapeutic approaches to limit post-MI LV remodeling in patients center on early reperfusion and neurohormonal blockade. The results of Hilgendorf et al. strongly support a new approach in acute MI that focuses on the temporal modulation of macrophage polarity toward a reparative phenotype. Macrophage phenotypic switching was observed to be a local phenomenon suggesting that the endogenous factors driving the switch were derived from the cardiac microenvironment. The key questions remain as to what are the local factors regulating Ly6Clow macrophage polarization in the infarcted heart, and how are they influenced, for example, by reperfusion and current pharmacological strategies. Identification of the endogenous drivers of Ly6Clow macrophage differentiation would potentially allow their pharmacological (or biological) targeting at circumscribed time points after MI in an effort to produce more efficacious wound healing and ameliorate remodeling. This indirect approach might be particularly appealing as the hormone receptor Nr4a1, the focus of the current study, has essential biological functions beyond macrophages; hence directly targeting Nr4a1 may yield unexpected, and perhaps undesirable, extracardiac effects.
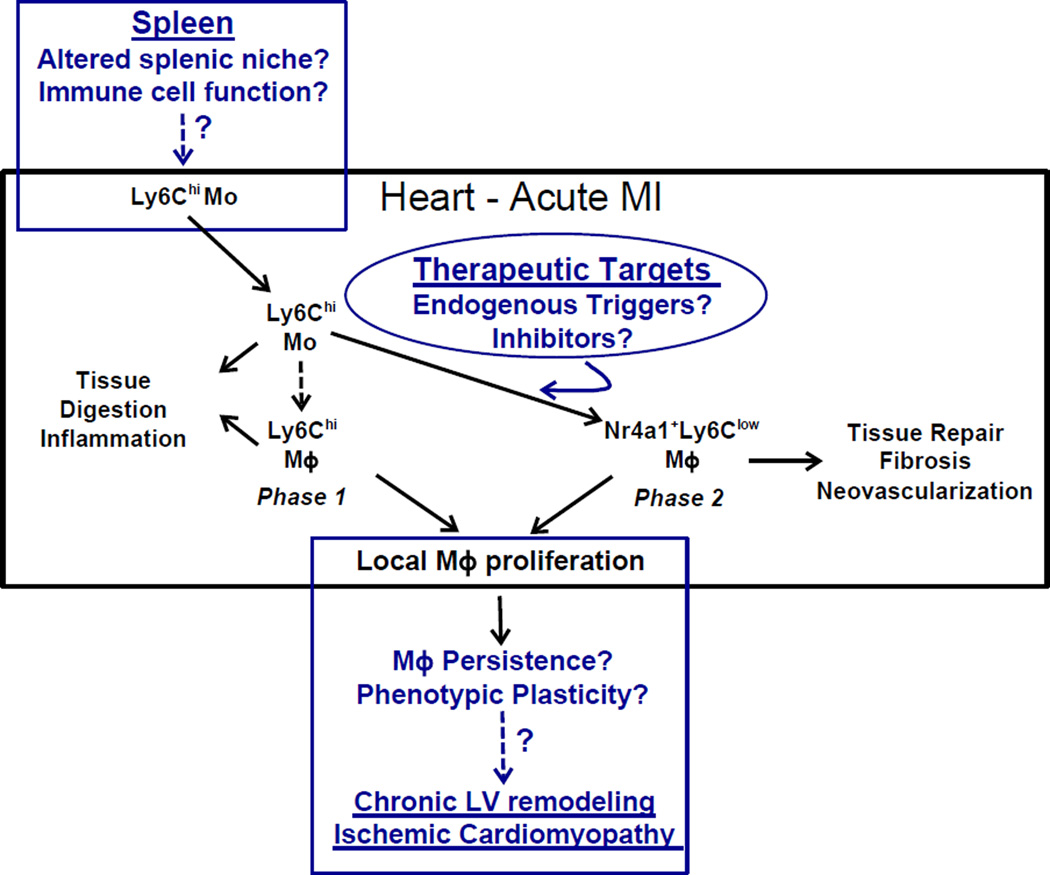
Figure.
The center box, in black, summarizes the results of the study by Hilgendorf et al. and prior work from the authors regarding two phases of monocyte (Mo) and macrophage (Mϕ) infiltration into the heart after myocardial infarction (MI). Both the initial pro-inflammatory Ly6Chi monocyte (and macrophage) dominant phase (peak ~3 d) and the delayed reparative Ly6Clow macrophage phase (peak ~7 d) derive from circulating Ly6Chi monocytes mobilized from the spleen, the latter phase triggered by the induction of Nr4a1 and indicative of phenotypic switching. Moreover, macrophages were shown to proliferate locally in the remodeling heart. The boxes in blue put forth three conceptual/translational implications raised by these studies related to: 1) the identification (and potential therapeutic targeting) of the local environmental factors, as of yet unidentified, that trigger reparative macrophage polarization, 2) the potential impact of sustained local macrophage proliferation and persistence on the pathogenesis of late remodeling and ischemic cardiomyopathy, and 3) the effect of alterations in splenic niches and/or immune cells on the tissue reparative process. See text for details and further discussion.
In addition to these translational implications, the demonstration of local macrophage differentiation and replenishment in the post-MI heart conceptually informs potential immune cell-mediated mechanisms of late ventricular remodeling post-MI. While the infiltration of pro-inflammatory monocytes and macrophages post-MI diminishes significantly after ~14 d in mice, recent studies in chronic ischemic murine heart failure (56 d post-MI) indicate that there is a local (and global) re-expansion of pro-inflammatory macrophages and dendritic cells that have detrimental effects on remote zone remodeling in the failing heart.22 The demonstration of ongoing and substantial local proliferation, and phenotypic plasticity, of macrophages in the infarcted heart by Hilgendorf et al. suggests a potential source within the remodeling heart for the late reemergence of M1 macrophages, and raises the intriguing possibility that limiting ongoing macrophage proliferation in the failing heart, well after the completion of infarct zone healing, may limit late remodeling in ischemic cardiomyopathy.
Lastly, these studies suggest a need for further definition of the role of the splenic microenvironment, and the cardiosplenic axis, in post-MI remodeling. Prior studies by these authors have indicated that the spleen is a primary source of monocytes mobilized to the acutely infarcted heart;7, 9 hence, it is spleen-derived Ly6Chi monocytes that help drive the cardiac-localized biphasic macrophage response described above. Conceivably, pathological (or pharmacological) alterations in splenic niches and/or splenic immune cells can impact monocyte function and/or mobilization (e.g., see reference 15) in a manner that interferes with the proper orchestration of remote phasic macrophage responses. Indeed, in chronic ischemic heart failure, the spleen undergoes intense white pulp remodeling and exhibits more prominent pro-inflammatory features, such that activated splenocytes that home to the heart induce fibrosis and tissue damage.22 However, whether specifically limiting splenic LyC6hi monocyte mobilization beyond the early post-MI period modulates subsequent long-term pathological remodeling is unknown.
In summary, the study by Hilgendorf et al. significantly informs and advances our understanding of the derivation and roles of the monocyte and macrophage phenotypes in the infarcted heart and its relationship to post-MI remodeling. By using chimeric Nr4a1−/− mice, and stringent flow cytometry and fate-mapping strategies, they have identified Nr4a1 as a potential molecular regulator of reparative macrophage differentiation and have established that macrophages proliferate and replenish locally in the infarcted heart. Their study adds to the growing body of evidence supporting the signal importance of macrophages, and other immune cell types, in the genesis of both early and late remodeling after MI, and the give further credence to the concept that immune cell modulation represents a novel therapeutic avenue for both improving cardiac repair and alleviating heart failure.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH grants HL-99014 and HL-78825, and a VA Merit Award.
Footnotes
DISCLOSURES
There are no relevant conflicts of interest.
REFERENCES
- 1.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 2.Prabhu SD. Post-infarction ventricular remodeling: An array of molecular events. J Mol Cell Cardiol. 2005;38:547–550. doi: 10.1016/j.yjmcc.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87:755–763. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 5.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Ismahil MA, Prabhu SD. Cardiac immune cell remodeling after myocardial infarction. J Mol Cell Cardiol. 2013;62:142–143. doi: 10.1016/j.yjmcc.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 11.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, Tricot B, Wojtkiewicz G, Dutta P, Sager HB, Borodovsky A, Novobrantseva T, Klebanov B, Fitzgerald K, Anderson DG, Libby P, Swirski FK, Weissleder R, Nahrendorf M. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, Sugano Y, Takahashi T, Abe H, Mochizuki S, Sano M, Yoshikawa T, Okada Y, Koyasu S, Ogawa S, Fukuda K. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 2012;125:1234–1245. doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 15.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 17.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilgendorf I, Gerhardt L, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherrer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor Nr4a1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–2825. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]