Abstract
Considerable research effort in the past several decades has focused on the impact of psychological stress, and stress hormones, on cancer progression. Numerous studies have reported that stress hormone treatment or in vivo stress exposure can enhance the growth of tumor cell lines in vitro, as well as tumors in animal models, and have begun to explore molecular mechanisms. Comparatively little research has focused on the impact of psychological stress and stress hormones on cancer initiation, in part due to inherent methodological challenges, but also because potential underlying biological mechanisms have remained obscure. In this review, we present a testable theoretical model of pathways by which stress may result in cellular transformation and tumorigenesis. This model supports our overarching hypothesis that psychological stress, acting through increased levels of catecholamines and/or cortisol, can increase DNA damage and/or reduce repair mechanisms, resulting in increased risk of DNA mutations leading to carcinogenesis. A better understanding of molecular pathways by which psychological stress can increase the risk of cancer initiation would open new avenues of translational research, bringing together psychologists, neuroscientists, and molecular biologists, potentially resulting in the development of novel approaches for cancer risk reduction at the population level.
Introduction
Among the general public, there is widespread belief that psychological stress can contribute to an increased risk of cancer, or even be a cause of cancer by itself (Willcox, Stewart, & Sitas, 2011). Within the scientific community however, there is considerable skepticism about a role for stress in the etiology of cancer. Significant associations between psychological stress and the incidence of various types of cancer have been reported and corroborated by meta-analysis (Chida & Steptoe, 2008). However, findings are mixed, and caution has been urged regarding the interpretation of positive associations in this literature (Chida, Hamer, Wardle, & Steptoe, 2008; Coyne, Ranchor, & Palmer, 2010; Nakaya, 2014). Research on this topic is methodologically challenging even in animal models. As with studies of stress effects on other diseases, the absence of a universally accepted definition of stress makes it difficult to interpret differing findings across studies. The generally accepted characterization of stress as occurring when environmental challenges exceed the individual’s ability to cope with the “stressor” underscores the complexity of assessing this construct, which can be characterized in terms of environmental (stressors), psychological (distress), and/or biological (catecholamines, cortisol) measures (Koolhaas et al., 2011). Cross-sectional, case–control study designs that are the mainstay of much epidemiological research seeking to explore risk factors for cancer are problematic for studies of stress effects. Bias in the recall of past exposure to stressors, and/or one’s ability to cope with those challenges, can be introduced by heightened current levels of stress associated with a cancer diagnosis and treatment, or as a result of the respondent’s belief in stress as a cause of cancer (Chida & Steptoe, 2008). Prospective studies avoid recall bias in stress assessment, but confront methodological challenges associated with not knowing the critical timing of the exposure to stress relative to the multistep etiology of cancer and the typically multiyear time course between initiation of tumorigenesis and clinically detectable disease (Colditz, Wolin, & Gehlert, 2012; Hanahan & Weinberg, 2011).
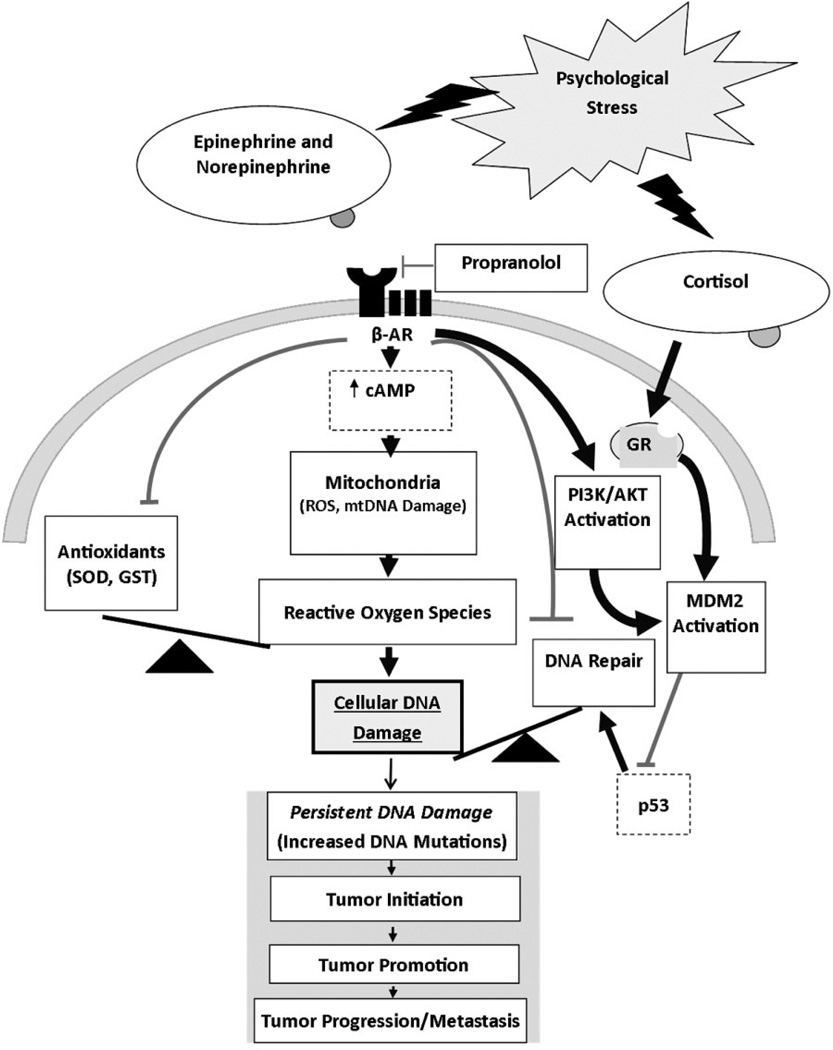
An additional major impediment to progress in research on stress effects in the etiology of cancer is the absence of well-established biological pathways that could provide causal linkages between the experience of stress and the development of cancer. Although multiple possible molecular pathways have been proposed for stress effects on the progression of existing cancer and are beginning to be tested (Costanzo, Sood, & Lutgendorf, 2011), proposed pathways for stress effects on cancer etiology have largely focused on possible immunological influences for more than two decades (Bovbjerg, 1991). In the absence of a testable theoretical model of pathways by which stress could contribute to cancer initiation at the molecular level, the investigation of stress effects on cancer etiology is likely to remain “black box” science (Weed, 1998) and have little impact on the broader biomedical literature. In this article, we put forward a testable theoretical model by which stress can not only get “under the skin” but also “reach into the heart” of the cell to affect processes that can result in the initiation of cancer (Figure 1). This model reflects our overarching hypothesis that psychological stress, acting through systemically increased levels of catecholamines and/or cortisol, can increase cancer risk through effects on DNA damage and repair mechanisms at the molecular level, which, in turn, can lead to mutations and carcinogenesis.
Figure 1.
Empirically based theoretical model of stress-induced effects on DNA damage and/or repair processes as biological mechanisms linking psychological stress to cancer risk. Heavy black lines indicate hypothesized stimulatory effects. Lighter gray lines indicate hypothesized inhibitory effects. β-AR—β-adrenergic receptor; GR—glucocorticoid receptor.
DNA damage, if not rectified by DNA repair mechanisms prior to cellular replication, can result in irreversible mutations that accumulate across repeated cell divisions and increase the risk of carcinogenesis (van Loon, Markkanen, & Hubscher, 2010). Underscoring the importance of DNA repair mechanisms, somatic DNA damage occurs frequently, with estimates of 20,000 lesions per cell on a daily basis (Markkanen, Hubscher, & van Loon, 2012). Much of that damage is caused by endogenous exposure to reactive oxygen species (ROS), largely produced in the cell’s own mitochondria as a by-product of cellular respiration (Bolisetty & Jaimes, 2013; Markkanen et al., 2012; Maynard, Schurman, Harboe, de Souza-Pinto, & Bohr, 2009). The most common oxidative DNA damage is caused by 8-hydroxylation of guanine, which is the most vulnerable of the four nucleobases in DNA due to its low redox potential (Poulsen, Nadal, Broedbaek, Nielsen, & Weimann, 2014). Protective mechanisms within the cell include antioxidant mechanisms (Rigoulet, Yoboue, & Devin, 2011; Sedelnikova et al., 2010), DNA repair mechanisms (Ciccia & Elledge, 2010; Jackson & Bartek, 2009), apoptotic mechanisms (Schmitt, Paquet, Beauchemin, & Bertrand, 2007), and the tumor suppressor protein p53 (Liu, Chen, & St Clair, 2008), which are all critically important for mitigating the effects of endogenously induced oxidative DNA damage. The 8-hydroxylation of guanine oxidative modification is highly mutagenic since it can be misread as thymine at the time of DNA replication, resulting in C:G to A:T transversions, which are among the most frequent mutations seen in many common tumors (van Loon et al., 2010; Markkanen et al., 2012). Although the terminology to describe the base by-product that results from oxidative modification has varied over time and across investigators, 7,8-dihydro-8-oxo-guanine (8-oxo-G) and the related 8-hydroxy-2′-deoxyguanosine (8-OHdG) have been widely assessed in cells, plasma, and urine as biomarkers of oxidative DNA damage and explored as risk factors for cancer development (Valavanidis, Vlachogianni, & Fiotakis, 2009). In case–control studies, urinary levels of 8-oxo-G or 8-OHdG (reflecting body-wide DNA damage) have been consistently found to be elevated among patients with various types of cancer (Kryston, Georgiev, Pissis, & Georgakilas, 2011). Recent prospective cohort studies, which rule out possible reverse causal relationships (cancer causing oxidative DNA damage), have found that high urinary levels of 8-oxo-G or 8-OHdG are a risk factor for subsequent development of several common cancers (Loft, Olsen, Moller, Poulsen, & Tjonneland, 2013; Loft et al., 2012). As one would expect given the dynamic balance between DNA damage and repair, there is also accumulating evidence relating reduced DNA repair capacity to an increased risk of developing cancer (Alberg et al., 2013; Paz-Elizur et al., 2008; Wang et al., 2012). To date, the effects of psychological stress have received scant attention in this biomedical literature.
Experimental Evidence Supporting Links Between Cancer Risk, Stress, and DNA Damage
Several reviews of epidemiological and clinical studies have reported an association between stressful life events and increased progression of cancers (Antoni et al., 2006; Chida et al., 2008). In addition, numerous studies have investigated associations between stress and cancer progression and metastasis using laboratory stress models in animals and demonstrated that stress increases the growth rate of existing tumors and increases the risk of tumor spread (metastasis) (Al-Wadei, Al-Wadei, Ullah, & Schuller, 2012; Al-Wadei, Plummer, & Schuller, 2009; Al-Wadei, Plummer, et al., 2012; Flint et al., 2013; Landen et al., 2007; Schuller, Al-Wadei, Ullah, & Plummer, 2012; Shahzad et al., 2010; Sklar & Anisman, 1980; Sood et al., 2006). A recent review and meta-analysis reported that “stress-related psychosocial factors” are associated with higher cancer incidence in initially healthy samples (n = 165 studies), poorer survival in cancer patient samples (n = 330 studies), and increased mortality in community samples (n = 53 studies) with any type of cancer (Chida et al., 2008). Although the literature linking stress to the initiation of cancer is smaller than that linking stress to progression of existing cancer, epidemiological studies have pointed to an association between the risk of developing a variety of cancers and prior experience of stressful life events and depression (Kang, 2002; Sklar & Anisman, 1981). The mechanisms responsible for links between stress and the initiation of cancer have yet to be elucidated.
We hypothesize that psychosocial stress and attendant increased production of ROS (Baum & Posluszny, 1999; Kihara, Teshima, Sogawa, & Nakagawa, 1992) can result in DNA damage and thus increase the likelihood of mutations that can lead to cancer. We further hypothesize that stress can result in suppression of DNA repair mechanisms, resulting in a “double whammy” of increased damage and decreased repair during times of stress. In support of this hypothesis are a considerable number of studies documenting the effects of psychological stress on DNA damage (reviewed by Cwikel, Gidron, & Quastel, 2010; Gidron, Russ, Tissarchondou, & Warner, 2006). For example, it has been reported in animal models that rotational stress impairs repair mechanisms for chemical carcinogen-induced DNA damage (Glaser, Thorn, Tarr, Kiecolt-Glaser, & D’Ambrosio, 1985) and psychological stress increases DNA damage as measured by levels of 8-OHdG (a marker of oxidative DNA damage) (Adachi, Kawamura, & Takemoto, 1993). In human studies, positive associations have been found between levels of 8-OHdG and a variety of psychological factors, including anxiety, depression, hostility, fatigue, and confusion (Carroll et al., 2010; Irie et al., 2001).
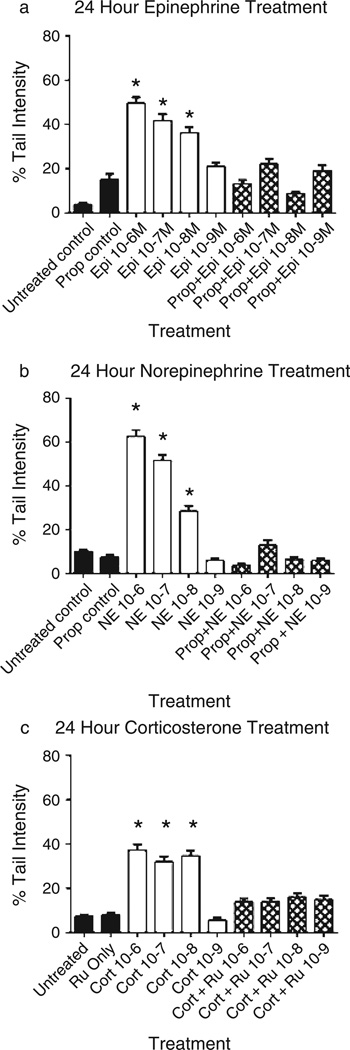
In more recent experimental studies, we have shown that in vitro treatment of NIH 3T3 cells (an immortalized mouse fibroblast line) with epinephrine or norepinephrine either short term (10 minutes) (Flint, Baum, Chambers, & Jenkins, 2007) or long term (24 hours) (Flint et al., 2013) results in significant DNA damage as measured by the alkaline comet assay. The alkaline comet assay, a well-validated and widely used single cell assay of DNA damage, uses a gel electrophoresis technique to pull cellular DNA fragments into a comet “tail” that can be quantified vis-à-vis undamaged DNA (percent tail intensity) (Collins & Azqueta, 2012). The comet assay is increasingly the method of choice in environmental and occupational biomonitoring studies of humans potentially exposed to carcinogens, as well as in basic science investigations (Azqueta & Collins, 2013; Valverde & Rojas, 2009). Figure 2a & b shows the comet DNA damage results following 24-hour exposure of NIH 3T3 cells to epinephrine or norepinephrine, whereas Figure 2c shows the results following exposure to corticosterone. Confirming receptor specificity, pretreatment of the cells with the β-adrenergic receptor antagonist propranolol blocked the catecholamine-induced increase in DNA damage, whereas pretreatment with the corticosterone receptor antagonist RU486 blocked the effects of corticosterone. The DNA damage produced by 24-hour exposure to either catecholamine resulted in mutations as evidenced by significant increases in cellular transformation phenotype (as measured by growth in soft agar) and these cells exhibited a more aggressive tumor growth in nude mice (Flint et al., 2013). These results are consistent with a growing environmental and occupational exposure literature that uses the transformation of the 3T3 line as a sensitive test for potential mutagens or carcinogens (Tanaka et al., 2012). Overall, our results suggest that stress (as modeled by exposure to stress hormones) can result in DNA damage and increased likelihood of initiation of a transformed phenotype and tumorigenesis.
Figure 2.
Effect of 24-hour exposure to stress hormones on DNA damage. NIH 3T3 cells were incubated in the presence or absence of (a) epinephrine, (b) norepinephrine, or (c) corticosterone for 24 hours and DNA damage measured by the alkaline comet assay. In some experiments, cells were pretreated for 30 minutes with 10−6M propranolol or RU486 prior to treatment with the stress hormones. Results are expressed as mean % tail intensity ± SD. * indicates significantly increased compared to untreated control (p < .05) using one-way ANOVA with Tukey’s post hoc analyses. Each experiment was performed twice and 100 cells analyzed in each comet assay. Pro—propranolol; RU—RU486; Epi—epinephrine; NE—norepinephrine; Cort—corticosterone (Flint et al., 2013; Jenkins & Bovbjerg, unpublished data).
Two recent studies by others using in vivo and in vitro models have further supported the hypothesis that stress can result in increased DNA damage. Hara et al. (2011) reported that stress could induce DNA damage and reduce p53 levels via the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway and β-arresting-1 activated pathway. This study suggests that one mechanism of catecholamine-induced increase in DNA damage is by modulation of p53 levels. In a related study, Feng et al. (2012) reported that increased levels of glucocorticoids produced as a result of restraint stress resulted in decreased p53 function, leading to tumorigenesis in heterozygous p53+/− mice irradiated with 44 Gy of ionizing radiation.
Proposed Model for the Role of Stress Hormones in the Production of DNA Damage
Based on the results from our laboratory and others (as outlined earlier), we have developed a theoretical model for the biological links between stress and increased DNA damage (Figure 1). According to this model, activation of β2-adrenergic receptors by catecholamines as a result of stress results in the production of cAMP and activation of PKA, which leads to increased oxidative phosphorylation and the production of ROS. ROS production can also be increased by β2-adrenergic receptor activation through nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. The increased production of ROS overwhelms the normal protective effect of antioxidants in the cell, resulting in increased DNA damage. In addition, β2-adrenergic receptor activation can also lead to activation of the phosphoinositide-3-kinase/Akt pathway, which increases MDM2 activation resulting in loss of p53 function. By reducing the availability of functional p53, DNA repair is negatively impacted and DNA lesions created by either errors in normal cellular replication or by ROS production become permanent. The glucocorticoids, cortisol (in humans) or corticosterone (in mice and rats), also decrease p53 function by activating MDM2. The introduction of permanent or persistent DNA damage increases the risk of cellular transformation and ultimately cancer formation. Each of the steps in these hypothesized pathways linking stress to cancer development in this model is briefly reviewed below.
β-Adrenergic and Glucocorticoid Stimulation
Adrenergic Receptors and Neurotransmitters
The autonomic nervous system controls a wide range of bodily functions, including heart rate, digestion, respiration, and perspiration (Chrousos, 2009). The “fight or flight” response is controlled by the sympathetic nervous system, part of the autonomic system. The stress hormones responsible for this response are the catecholamines epinephrine and norepinephrine and ultimately cortisol (corticosterone in rodents).
The production of epinephrine is powerfully affected by different types of environmental stressors, such as physical threats and excitation. These stressors activate the central nervous system to send signals to the chromaffin cells in the adrenal gland, resulting in the production and release of epinephrine into the blood stream. Norepinephrine is produced both by the adrenal gland (where it is released into the blood stream) and by the sympathetic ganglionic neurons in effector organs. Both epinephrine and norepinephrine bind to adrenergic receptors.
Adrenergic receptors are among the most well-studied G protein-coupled receptors and are located in cellular membranes. They contain a typical seven-transmembrane region along with extracellular and intracellular domains. There are two main types of adrenergic receptors, α and β, which can be further divided into nine subsets (α1A, α1B, α1D, α2A, α2B, α2C, β1, β2, and β3 (Katritch, Cherezov, & Stevens, 2013). The different types of adrenergic receptors overlap in terms of location and function. For example, both α- and β-receptors are present in various smooth muscles, such as arterial wall, uterus, and adipose tissue. In uterine smooth muscle, both types act as a vasoconstrictor when activated, whereas in arteriolar smooth muscle, the α-receptors act as vasoconstrictors in visceral organs and the β2-receptor acts as a vasodilator in skeletal muscle and liver (Gomperts, Kramer, & Tatham, 2002). Activation of α-adrenoreceptors generally suppresses cAMP production, whereas activation of the β-adrenoreceptors induces cAMP production (Gomperts et al., 2002; Hutchinson, Chernogubova, Dallner, Cannon, & Bengtsson, 2005).
Glucocorticoids
Cortisol is produced by the adrenal gland as part of the hypothalamic–pituitary–adrenal (HPA) axis. During a stressful event, the HPA axis is activated, resulting in signals being sent to the paraventricular nucleus of the hypothalamus, which produces corticotropin-releasing hormone (CRH). CRH acts on the corticotrope cells of the pituitary to produce and release adrenocorticotropic hormone (ACTH) into the blood stream. ACTH stimulates the adrenal cortex to synthesize cortisol in humans and corticosterone in mice (Armario et al., 2012).
Downstream Signaling of the β2-Adrenoreceptor
Binding of epinephrine, norepinephrine, or an agonist (such as isoproterenol) to the β2-receptor results in a conformational change of the receptor, leading to the production of cAMP via adenyl cyclase. Increased levels of cAMP result in the activation of PKA, which, in turn, initiates downstream effects, including the mobilization of calcium ions (Rockman, Koch, & Lefkowitz, 2002) and phosphorylation of the cAMP-responsive element binding protein/activating transcription factor family of transcription factors (Cole & Sood, 2012). Stimulation of the β2-receptor also leads to binding by β-arrestin, which causes endocytosis of the receptor and activation of the mitogen-activated protein kinase (MAPK) pathway (Kendall et al., 2011; Rockman et al., 2002; Shenoy & Lefkowitz, 2011).
Activation of the β2-receptor has also been reported to increase cellular ROS production. Transgenic mice overexpressing the β2-receptor were shown to have higher levels of ROS and activated MAPK pathway compared with non-transgenic littermates. Treatment of the mice with antioxidants or NADPH oxidase inhibitors resulted in lower ROS production, suggesting that β2-receptor activation results in NAPDPH oxidase-induced ROS (Xu et al., 2011). In a separate study, treatment of murine cardiomyocytes with the β-adrenergic agonist isoproterenol increased mitochondrial ROS production in a cAMP-PKA-dependent manner (Andersson et al., 2011).
Taken together, it is clear that β-adrenergic receptors (especially β2) are present on a number of different tissues and cells in the body, and activation of these receptors by the catecholamines epinephrine and norepinephrine have a wide variety of effects, including increased ROS production and activation of intracellular signaling pathways such as the MAPK pathway.
Downstream Signaling of the Glucocorticoid Receptor
Cortisol (and corticosterone) binds to the glucocorticoid receptor (GR). The GR is found in an inactivated form in the nucleus in a complex with several proteins, including heat-shock proteins 90 and 70. Upon binding of glucocortoid hormone to GR, the receptor/ligand complex is released from hsp90 allowing for dimerization and nuclear translocation of the complex followed by DNA binding and transactivation of glucocorticoid-responsive genes (Heitzer, Wolf, Sanchez, Witchel, & DeFranco, 2007; Pratt, 1990). This transactivation can result in either up-regulation or down-regulation of individual genes. Thus, unlike the β2-receptor, which, upon activation by binding with a catecholamine, results in a second messenger signal system, binding of the GR by a glucocorticoid results in direct action on glucocorticoid-responsive gene transcription.
Oxidation phosphorylation and ROS production
Cellular energy is produced by glycolysis and oxidative phosphorylation with ROS as a by-product (Figueira et al., 2013). During glycolysis, glucose is converted into two molecules of pyruvate with the net production of two molecules of ATP. This pyruvate has two cellular fates. First, under hypoxic conditions, pyruvate can be converted into lactate and transported out of the cell by the monocarboxylate transporters. Alternatively, pyruvate can be metabolized into acetyl-coA by pyruvate dehydrogenase in the mitochondria, where it can travel through the tricarboxylic acid (TCA) cycle to generate (see Figure 3). Glucose is widely used as an energy source, but different tissues can use various carbon sources to generate ATP, including pyruvate, glutamine, lactate, and fatty acids. The heart, e.g., generates 50%–70% of its total energy from fatty acid β-oxidation in the mitochondria. As first noted by Randle, Garland, Hales, and Newsholme (1963), tissues that utilize glucose do not normally utilize fatty acids for energy, and the reverse is also true (Hue & Taegtmeyer, 2009). However, under times of stress and release of epinephrine, the heart can actually utilize both carbon sources simultaneously (Collins-Nakai, Noseworthy, & Lopaschuk, 1994; Depre, Ponchaut, Deprez, Maisin, & Hue, 1998).
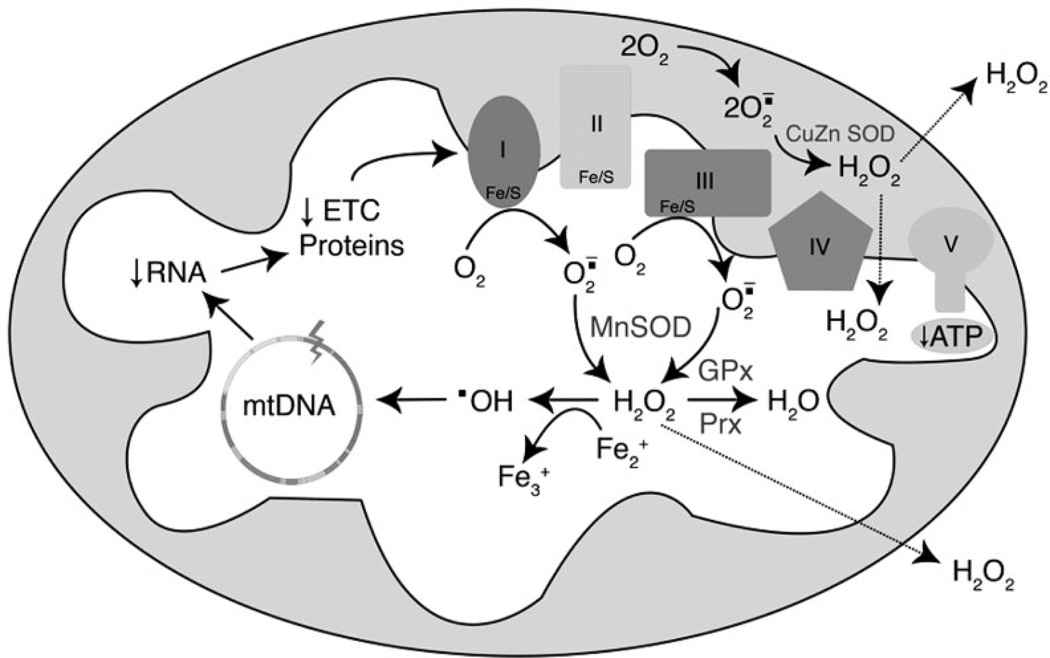
Figure 3.
Mitochondrial oxidative phosphorylation and ROS production. NADH and FADH2 are produced in the TCA cycle, which donates electrons to the electron transport chain (ETC). During mitochondrial respiration, a small amount of the molecular oxygen consumed by cells is converted into superoxide anion radical (O2.) by complexes I and III. Superoxide dismutase (SOD) enzymes (MnSOD and CuZn SOD) convert O2 to hydrogen peroxide (H2O2), which can be sequentially converted into H2O by glutathione peroxidase (GPx) or peroxiredoxin (Prx) enzymes. Also, H2O2 can have a different fate if it reacts with Fe2+, in this case generating hydroxyl radical (.OH). This radical can attack all molecules including mtDNA and consequently cause a decrease in mitochondrial mRNA and altered expression of mitochondrial proteins essential for ETC and ATP synthesis. Defects in mitochondrial proteins affect ETC activity culminating in a vicious cycle of ROS production.
NADH and FADH2 can donate electrons to the electron transport chain (ETC) at complex I or complex II, respectively, which feed electrons to ubiquinone, complex III, and finally complex IV where oxygen is converted into water through a four-electron reduction. Simultaneously with the movement of electrons along the ETC, protons are pumped from the matrix into the inter-membrane space at complexes I, III, and IV to generate a proton gradient. This proton gradient is harvested by complex V, ATP synthase, a nanomachine that spins and couples the flow of protons back into the matrix to synthesize ATP from ADP and Pi. Mitochondrial oxidative phosphorylation generates a net production of 31 ATP molecules. Traditionally, this number has been given as 38 ATP molecules based on a whole number of P/O ratios of NADH and FADH2 of three and two, respectively. However, these numbers have been revised to 2.5 and 1.5 for NADH and FADH2, respectively, thus yielding a total of 31 molecules for the complete oxidation of glucose.
As electrons are carried across the electron transport chain, some are captured by oxygen to produce superoxide anion radicals (O2.) primarily at complex I and complex III (Brandon, Baldi, & Wallace, 2006). Careful analysis of the topology of where superoxide is generated has indicated that as much as 30% of the superoxide generated at complex III is in the inner membrane space, whereas 70% is generated in the matrix (St-Pierre, Buckingham, Roebuck, & Brand, 2002). Superoxide radical anion can attack FeS clusters in subunits of the electron transport chain and in the TCA cycle (aconitase), liberating iron and causing inactivation of the proteins. These superoxide radicals are converted into hydrogen peroxide by the action of manganese superoxide dismutase (SOD2) within the matrix and copper zinc superoxide dismutates (SOD1) in the intermembrane space. Hydrogen peroxide in the mitochondria is broken down to water by the action of glutathione peroxidase or peroxiredoxins. Former estimates of hydrogen peroxide were based on isolated highly energized mitochondria and suggested that as high as 2%–4% of the oxygen consumed by mitochondria is liberated as superoxide or hydrogen peroxide (Boveris, Oshino, & Chance, 1972). More recent studies on whole cells suggest that these early estimates are one to two orders of magnitude too high (Murphy, 2009; St-Pierre et al., 2002). Since superoxide is negatively charged, it cannot cross the inner mitochondrial membrane, whereas hydrogen peroxide is freely diffusible and can attack all macromolecules in the cell, including nuclear DNA. Hydrogen peroxide is not very reactive, but in the presence of divalent metal ions such as reduced iron, it is converted into the highly reactive hydroxyl radical, through Fenton chemistry. The hydroxyl radical is highly reactive and will attack any molecule within a few bond lengths, including biomolecules of mitochrondrial (mt)DNA, lipids, proteins, or even water. In addition to the enzymatic neutralization of free radicals in the mitochondria, several compounds have been shown to mitigate the oxidant injury, these include glutathione, vitamins C and E, and ubiquinone.
ROS damage to DNA
Hydroxyl attack of DNA in the mitochondria and the nucleus can produce a wide spectrum of DNA lesions, including single- and double-strand breaks, base loss, and base damage such as 8-oxo-dG and thymine glycol. Base damage in either nuclear or mtDNA damage is repaired by base excision repair (Sawyer & Van Houten, 1999). mtDNA is more susceptible to oxidative damage than nuclear DNA (Furda, Bess, Meyer, & Van Houten, 2012; Santos, Hunakova, Chen, Bortner, & Van Houten, 2003; Yakes & Van Houten, 1997). We and others have proposed that chronic mtDNA damage causes a vicious cycle of ROS production and serves to amplify oxidant injury during disease (Furda et al., 2012; Van Houten, Woshner, & Santos, 2006; Yakes & Van Houten, 1997). Damaged mtDNA causes a decrease in transcription and the synthesis of the 13 polypeptides encoded in the mtDNA, which are associated with the electron transport and complex V. This inhibition of ETC proteins can cause a subsequent increase in ROS, resulting in a decrease in the mitochondrial membrane potential, loss of ATP and energy collapse, and subsequent cell death (Simsek et al., 2011; Tann et al., 2011). Long-lived DNA strand breaks in mtDNA due to loss of key DNA repair enzymes can result in apoptotic cell death. Thus, mitochondrial dysfunction and ROS are intimately linked in a large number of human pathologies (Van Houten et al., 2006).
DNA damage response
Nuclear DNA damage resulting from oxidative stress can result in a robust response involving a large number of proteins. Central to this damage response is p53, a transcription factor that activates the expression of a wide range of genes involved in different cellular processes, including DNA repair, programmed cell death (apoptosis), and metabolism (Gottlieb & Vousden, 2010; Menendez, Inga, & Resnick, 2009; Ryan & Vousden, 2002; Vousden & Ryan, 2009). Normally, p53 protein is bound by MDM2, which causes p53 to be rapidly degraded. However, during DNA damage, MDM2 is phosphorylated and no longer binds to p53, which is then stabilized. p53 has both nuclear (Menendez et al., 2009) and cytoplasmic roles (Chipuk & Green, 2004; Green & Kroemer, 2009), and under persistent damage can transcriptionally induce a number of pro-apopotic factors and also bind to mitochondria to promote cell death.
Summary and Future Directions
The integrity of the human genome is continually threatened by exogenous (e.g., ultraviolet radiation from the sun) and endogenous (e.g., ROS produced as a by-product of normal cellular metabolism) challenges that, if not kept in check by protective mechanisms (e.g., antioxidant activity, DNA repair), can result in increased damage to DNA and ultimately increased risk of cancer (Bernstein, Nfonsam, Prasad, & Bernstein, 2013; Cadet & Wagner, 2013; Hoeijmakers, 2009; Kryston et al., 2011; van Loon et al., 2010; Sedelnikova et al., 2010). A role for psychological stress as a contributor to increased DNA damage has been supported by correlational studies in humans (Cwikel et al., 2010; Gidron et al., 2006), as well as by experimental research with animals exposed to stress (Cwikel et al., 2010; Gidron et al., 2006) and cell lines exposed to stress hormones in vitro (Flint et al., 2005, 2007, 2013; Gidron et al., 2006; Hara et al., 2011). Critically lacking in the literature are experimental studies with humans to confirm a causal connection between psychological stress and increased DNA damage, despite the availability of well-established methods (e.g., the Trier Social Stress Test) to reliably elicit stress responses in humans under controlled laboratory conditions, and in vitro evidence that exposure to stress hormones for as little as ten minutes can result in increased DNA damage (Flint et al., 2007). Concurrent translational research with experimental stress in animal models is needed to explore molecular mechanisms by which stress-induced increases in DNA damage result in mutations that can lead to carcinogenesis (Flint et al., 2013).
Also currently lacking in the literature are experimental studies in humans and animal models to investigate the underlying mechanisms responsible for stress-induced increases in DNA damage at systemic and molecular levels. At the systemic level, in vivo research is needed to investigate the role of stress-induced neuroendocrine responses (e.g., increased circulating levels of catecholamines and cortisol) as instigators of stress-induced increases in DNA damage. At the molecular level, research is needed to determine the contribution of stress effects on the two major pathways whose net activity largely determines ongoing levels of DNA damage (Kryston et al., 2011; Sedelnikova et al., 2010): (a) psychological stress may cause an increase in ROS levels (Cwikel et al., 2010; Gidron et al., 2006), resulting in increased oxidative DNA damage due to an imbalance in the usual dynamic equilibrium between ROS and antioxidant activity (a state described as “oxidative stress”; Dizdaroglu, 2012; Sedelnikova et al., 2010); and/or (b) stress may cause a reduction in DNA repair capacity (Cwikel et al., 2010; Gidron et al., 2006), resulting in an imbalance in the usual dynamic equilibrium between DNA damage and repair such that the cell would not be able to keep up with ongoing challenges to DNA integrity (Jalal, Earley, & Turchi, 2011; Kryston et al., 2011).
Contributor Information
Frank J. Jenkins, Department of Pathology, Infectious Diseases and Microbiology, University of Pittsburgh and Biobehavioral Medicine in Oncology Program, University of Pittsburgh Cancer Institute.
Bennett Van Houten, Department of Chemical Biology and Pharmacology, University of Pittsburgh and Molecular and Cellular Cancer Biology Program, University of Pittsburgh Cancer Institute.
Dana H. Bovbjerg, Department of Psychiatry, Psychology, and Behavioral and Community Health Sciences, University of Pittsburgh and Biobehavioral Medicine in Oncology Program, University of Pittsburgh Cancer Institute
References
- Adachi S, Kawamura K, Takemoto K. Oxidative damage of nuclear DNA in liver of rats exposed to psychological stress. Cancer Research. 1993;53:4153–4155. [PubMed] [Google Scholar]
- Alberg AJ, Jorgensen TJ, Ruczinski I, Wheless L, Shugart YY, Berthier-Schaad Y, et al. DNA repair gene variants in relation to overall cancer risk: A population-based study. Carcinogenesis. 2013;34:86–92. doi: 10.1093/carcin/bgs304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wadei HA, Al-Wadei MH, Ullah MF, Schuller HM. Celecoxib and GABA cooperatively prevent the progression of pancreatic cancer in vitro and in xenograft models of stress-free and stress-exposed mice. PLoS One. 2012;7:e43376. doi: 10.1371/journal.pone.0043376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wadei HA, Plummer HK, 3rd, Schuller HM. Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acid. Carcinogenesis. 2009;30:506–511. doi: 10.1093/carcin/bgp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wadei HA, Plummer HK, 3rd, Ullah MF, Unger B, Brody JR, Schuller HM. Social stress promotes and gamma-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancer. Cancer Prevention Research (Philadelphia) 2012;5:189–196. doi: 10.1158/1940-6207.CAPR-11-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DC, Fauconnier J, Yamada T, Lacampagne A, Zhang SJ, Katz A, et al. Mitochondrial production of reactive oxygen species contributes to the beta-adrenergic stimulation of mouse cardiomycytes. The Journal of Physiology. 2011;589(Pt 7):1791–1801. doi: 10.1113/jphysiol.2010.202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PGK, et al. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A, Daviu N, Munoz-Abellan C, Rabasa C, Fuentes S, Belda X, et al. What can we know from pituitary-adrenal hormones about the nature and consequences of exposure to emotional stressors? Cellular and Molecular Neurobiology. 2012;32:749–758. doi: 10.1007/s10571-012-9814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azqueta A, Collins AR. The essential comet assay: A comprehensive guide to measuring DNA damage and repair. Archives of Toxicology. 2013;87:949–968. doi: 10.1007/s00204-013-1070-0. [DOI] [PubMed] [Google Scholar]
- Baum A, Posluszny DM. Health psychology: Mapping biobehavioral contributions to health and illness. Annual Review of Psychology. 1999;50:137–163. doi: 10.1146/annurev.psych.50.1.137. [DOI] [PubMed] [Google Scholar]
- Bernstein C, Nfonsam V, Prasad AR, Bernstein H. Epigenetic field defects in progression to cancer. World J Gastrointest Oncol. 2013;5:43–49. doi: 10.4251/wjgo.v5.i3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolisetty S, Jaimes EA. Mitochondria and reactive oxygen species: Physiology and pathophysiology. International Journal of Molecular Sciences. 2013;14:6306–6344. doi: 10.3390/ijms14036306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovbjerg DH. Psychoneuroimmunology. Implications for oncology? Cancer. 1991;67(3 Suppl):828–832. doi: 10.1002/1097-0142(19910201)67:3+<828::aid-cncr2820671413>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. The Biochemical Journal. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harbor Perspectives in Biology. 2013;5:1–18. doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Marsland AL, Jenkins F, Baum A, Muldoon MF, Manuck SB. A urinary marker of oxidative stress covaries positively with hostility among midlife community volunteers. Psychosomatic Medicine. 2010;72:273–280. doi: 10.1097/PSY.0b013e3181d0d72b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature Clinical Practice Oncology. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine. 2008;70:741–756. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. Cytoplasmic p53: Bax and forward. Cell Cycle. 2004;3:429–431. [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Molecular Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Science Translational Medicine. 2012;4:127rv124. doi: 10.1126/scitranslmed.3003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Sood AK. Molecular pathways: Beta-adrenergic signaling in cancer. Clinical Cancer Research. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR, Azqueta A. DNA repair as a biomarker in human biomonitoring studies; further applications of the comet assay. Mutation Research. 2012;736:122–129. doi: 10.1016/j.mrfmmm.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Collins-Nakai RL, Noseworthy D, Lopaschuk GD. Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. The American Journal of Physiology. 1994;267(5 Pt 2):H1862–H1871. doi: 10.1152/ajpheart.1994.267.5.H1862. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Sood AK, Lutgendorf SK. Biobehavioral influences on cancer progression. Immunology and Allergy Clinics of North America. 2011;31:109–132. doi: 10.1016/j.iac.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JC, Ranchor AV, Palmer SC. Meta-analysis of stress-related factors in cancer. Nature Reviews Clinical Oncology. 2010;7:1–2. doi: 10.1038/ncponc1134-c1. [DOI] [PubMed] [Google Scholar]
- Cwikel JG, Gidron Y, Quastel M. Low-dose environmental radiation, DNA damage, and cancer: The possible contribution of psychological factors. Psychology, Health and Medicine. 2010;15:1–16. doi: 10.1080/13548500903431493. [DOI] [PubMed] [Google Scholar]
- Depre C, Ponchaut S, Deprez J, Maisin L, Hue L. Cyclic AMP suppresses the inhibition of glycolysis by alternative oxidizable substrates in the heart. The Journal of Clinical Investigation. 1998;101:390–397. doi: 10.1172/JCI1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M. Oxidatively induced DNA damage: Mechanisms, repair and disease. Cancer Letters. 2012;327:26–47. doi: 10.1016/j.canlet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, et al. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7013–7018. doi: 10.1073/pnas.1203930109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JC, Kowaltowski AJ, et al. Mitochondria as a source of reactive oxygen and nitrogen species: From molecular mechanisms to human health. Antioxidants and Redox Signaling. 2013;18:2029–2074. doi: 10.1089/ars.2012.4729. [DOI] [PubMed] [Google Scholar]
- Flint MS, Baum A, Chambers WH, Jenkins FJ. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;32:470–479. doi: 10.1016/j.psyneuen.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Flint MS, Baum A, Episcopo B, Knickelbein KZ, Liegey Dougall AJ, Chambers WH, et al. Chronic exposure to stress hormones promotes transformation and tumorigenicity of 3T3 mouse fibroblasts. Stress. 2013;16:114–121. doi: 10.3109/10253890.2012.686075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint MS, Carroll JE, Jenkins FJ, Chambers WH, Han ML, Baum A. Genomic profiling of restraint stress-induced alterations in mouse T lymphocytes. Journal of Neuroimmunology. 2005;167:34–44. doi: 10.1016/j.jneuroim.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Furda AM, Bess AS, Meyer JN, Van Houten B. Analysis of DNA damage and repair in nuclear and mitochondrial DNA of animal cells using quantitative PCR. Methods in Molecular Biology. 2012;920:111–132. doi: 10.1007/978-1-61779-998-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidron Y, Russ K, Tissarchondou H, Warner J. The relation between psychological factors and DNA-damage: A critical review. Biological Psychology. 2006;72:291–304. doi: 10.1016/j.biopsycho.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Glaser R, Thorn BE, Tarr KL, Kiecolt-Glaser JK, D’Ambrosio SM. Effects of stress on methyltransferase synthesis: An important DNA repair enzyme. Health Psychology. 1985;4:403–412. doi: 10.1037//0278-6133.4.5.403. [DOI] [PubMed] [Google Scholar]
- Gomperts BD, Kramer IM, Tatham PER. Signal transduction. San Diego, CA: Academic Press; 2002. [Google Scholar]
- Gottlieb E, Vousden KH. p53 regulation of metabolic pathways. Cold Spring Harbor Perspectives Biology. 2010;2:a001040. doi: 10.1101/cshperspect.a001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature. 2011;477:349–353. doi: 10.1038/nature10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB. Glucocorticoid receptor physiology. Reviews in Endocrine and Metabolic Disorders. 2007;8:321–330. doi: 10.1007/s11154-007-9059-8. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. The New England Journal of Medicine. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Hue L, Taegtmeyer H. The Randle cycle revisited: A new head for an old hat. American Journal of Physiology. Endocrinology and Metabolism. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. Beta-adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia. 2005;48:2386–2395. doi: 10.1007/s00125-005-1936-7. [DOI] [PubMed] [Google Scholar]
- Irie M, Asami S, Nagata S, Ikeda M, Miyata M, Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Japanese Journal of Cancer Research. 2001;92:367–376. doi: 10.1111/j.1349-7006.2001.tb01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal S, Earley JN, Turchi JJ. DNA repair: From genome maintenance to biomarker and therapeutic target. Clinical Cancer Research. 2011;17:6973–6984. doi: 10.1158/1078-0432.CCR-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH. Oxidative stress, DNA damage, and breast cancer. AACN Clinical Issues. 2002;13:540–549. doi: 10.1097/00044067-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annual Review of Pharmacology and Toxicology. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall RT, Strungs EG, Rachidi SM, Lee MH, El-Shewy HM, Luttrell DK, et al. The beta-arrestin pathway-selective type 1A angiotensin receptor (AT1A) agonist [Sar1,Ile4,Ile8]angiotensin II regulates a robust G protein-independent signaling network. The Journal of Biological Chemistry. 2011;286:19880–19891. doi: 10.1074/jbc.M111.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara H, Teshima H, Sogawa H, Nakagawa T. Stress and superoxide production by human neutrophils. Annals of the New York Academy of Sciences. 1992;650:307–310. doi: 10.1111/j.1749-6632.1992.tb49142.x. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, et al. Stress revisited: A critical evaluation of the stress concept. Neuroscience and Biobehavioral Reviews. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutation Research. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Lin YG, Armaiz Pena GN, Das PD, Arevalo JM, Kamat AA, et al. Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Research. 2007;67:10389–10396. doi: 10.1158/0008-5472.CAN-07-0858. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen Y, St Clair DK. ROS and p53: A versatile partnership. Free Radical Biology and Medicine. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft S, Olsen A, Moller P, Poulsen HE, Tjonneland A. Association between 8-oxo-7,8-dihydro-2’-deoxyguanosine excretion and risk of postmenopausal breast cancer: Nested case-control study. Cancer Epidemiology, Biomarkers & Prevention. 2013;22:1289–1296. doi: 10.1158/1055-9965.EPI-13-0229. [DOI] [PubMed] [Google Scholar]
- Loft S, Svoboda P, Kawai K, Kasai H, Sorensen M, Tjonneland A, et al. Association between 8-oxo-7,8-dihydroguanine excretion and risk of lung cancer in a prospective study. Free Radical Biology and Medicine. 2012;52:167–172. doi: 10.1016/j.freeradbiomed.2011.10.439. [DOI] [PubMed] [Google Scholar]
- van Loon B, Markkanen E, Hubscher U. Oxygen as a friend and enemy: How to combat the mutational potential of 8-oxo-guanine. DNA Repair. 2010;9:604–616. doi: 10.1016/j.dnarep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Markkanen E, Hubscher U, van Loon B. Regulation of oxidative DNA damage repair: The adenine 8-oxo-guanine problem. Cell Cycle. 2012;11:1070–1075. doi: 10.4161/cc.11.6.19448. [DOI] [PubMed] [Google Scholar]
- Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nature Reviews. Cancer. 2009;9:724–737. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical Journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya N. Effect of psychosocial factors on cancer risk and survival. Journal of Epidemiology. 2014;24:1–6. doi: 10.2188/jea.JE20130124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Elizur T, Sevilya Z, Leitner-Dagan Y, Elinger D, Roisman LC, Livneh Z. DNA repair of oxidative DNA damage in human carcinogenesis: Potential application for cancer risk assessment and prevention. Cancer Letters. 2008;266:60–72. doi: 10.1016/j.canlet.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen HE, Nadal LL, Broedbaek K, Nielsen PE, Weimann A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochimica et Biophysica Acta. 2014;1840:801–808. doi: 10.1016/j.bbagen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Pratt WB. Glucocorticoid receptor structure and the initial events in signal transduction. Progress in Clinical and Biological Research. 1990;322:119–132. [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H(2)O(2) signaling. Antioxidants and Redox Signaling. 2011;14:459–468. doi: 10.1089/ars.2010.3363. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Ryan KM, Vousden KH. Cancer: Pinning a change on p53. Nature. 2002;419:795–797. doi: 10.1038/419795a. [DOI] [PubMed] [Google Scholar]
- Santos JH, Hunakova L, Chen Y, Bortner C, Van Houten B. Cell sorting experiments link persistent mitochondrial DNA damage with loss of mitochondrial membrane potential and apoptotic cell death. The Journal of Biological Chemistry. 2003;278:1728–1734. doi: 10.1074/jbc.M208752200. [DOI] [PubMed] [Google Scholar]
- Sawyer DE, Van Houten B. Repair of DNA damage in mitochondria. Mutation Research. 1999;434:161–176. doi: 10.1016/s0921-8777(99)00027-0. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Paquet C, Beauchemin M, Bertrand R. DNA-damage response network at the crossroads of cell-cycle checkpoints, cellular senescence and apoptosis. Journal of Zhejiang University Science B. 2007;8:377–397. doi: 10.1631/jzus.2007.B0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller HM, Al-Wadei HA, Ullah MF, Plummer HK., 3rd Regulation of pancreatic cancer by neuropsychological stress responses: A novel target for intervention. Carcinogenesis. 2012;33:191–196. doi: 10.1093/carcin/bgr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutation Research. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. The Journal of Biological Chemistry. 2010;285:35462–35470. doi: 10.1074/jbc.M110.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends in Pharmacological Sciences. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek D, Furda A, Gao Y, Artus J, Brunet E, Hadjantonakis AK, et al. Crucial role for DNA ligase III in mitochondria but not in Xrcc1-dependent repair. Nature. 2011;471:245–248. doi: 10.1038/nature09794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar LS, Anisman H. Social stress influences tumor growth. Psychosomatic Medicine. 1980;42:347–365. doi: 10.1097/00006842-198005000-00005. [DOI] [PubMed] [Google Scholar]
- Sklar LS, Anisman H. Stress and cancer. Psychological Bulletin. 1981;89:369–406. [PubMed] [Google Scholar]
- Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clinical Cancer Research. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. The Journal of Biological Chemistry. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Bohnenberger S, Kunkelmann T, Munaro B, Ponti J, Poth A, et al. Prevalidation study of the BALB/c 3T3 cell transformation assay for assessment of carcinogenic potential of chemicals. Mutation Research. 2012;744:20–29. doi: 10.1016/j.mrgentox.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Tann AW, Boldogh I, Meiss G, Qian W, Van Houten B, Mitra S, et al. Apoptosis induced by persistent single-strand breaks in mitochondrial genome: Critical role of exog (5’-exo/endonuclease) in their repair. The Journal of Biological Chemistry. 2011;286:31975–31983. doi: 10.1074/jbc.M110.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. Journal of Environmental Science and Health. Part C. Environmental Carcinogenesis & Ecotoxicology Reviews. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- Valverde M, Rojas E. Environmental and occupational biomonitoring using the comet assay. Mutation Research. 2009;681:93–109. doi: 10.1016/j.mrrev.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair. 2006;5:145–152. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Ryan KM. p53 and metabolism. Nature Reviews Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang M, Chen Y, Zhang Z, Wang S, Xu M, et al. The hOGG1 Ser326Cys polymorphism contributes to cancer susceptibility: Evidence from 83 case-control studies. Mutagenesis. 2012;27:329–336. doi: 10.1093/mutage/ger083. [DOI] [PubMed] [Google Scholar]
- Weed DL. Beyond black box epidemiology. American Journal of Public Health. 1998;88:12–14. doi: 10.2105/ajph.88.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox SJ, Stewart BW, Sitas F. What factors do cancer patients believe contribute to the development of their cancer? (New South Wales, Australia) Cancer Causes and Control. 2011;22:1503–1511. doi: 10.1007/s10552-011-9824-6. [DOI] [PubMed] [Google Scholar]
- Xu Q, Dalic A, Fang L, Kiriazis H, Ritchie RH, Sim K, et al. Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. British Journal of Pharmacology. 2011;162:1012–1028. doi: 10.1111/j.1476-5381.2010.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]