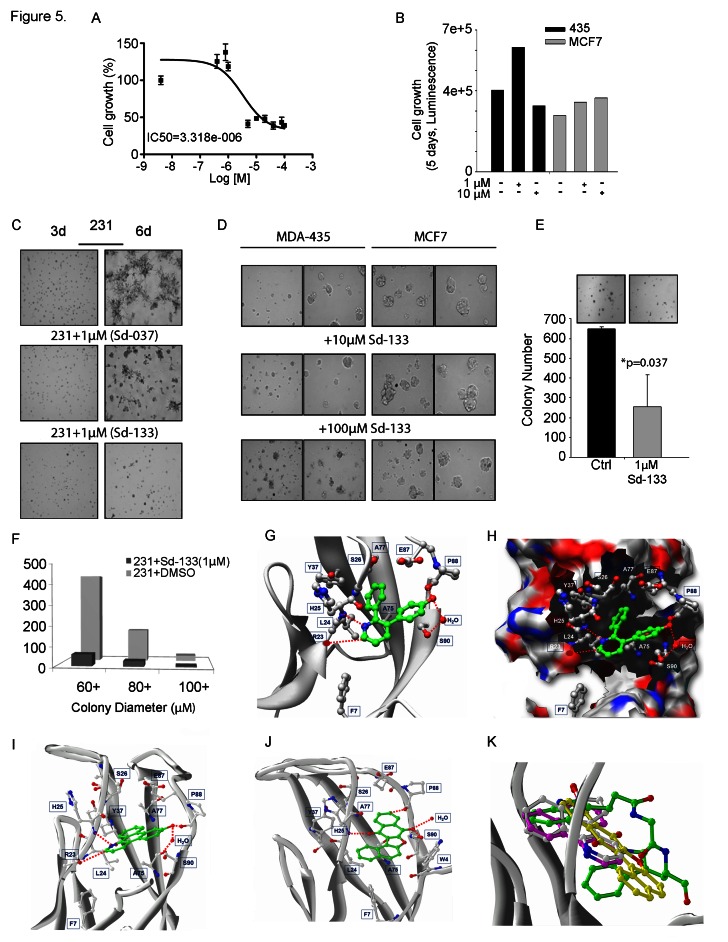
Figure 5. Development of small molecule inhibitors and their effect on CDH11 function-inhibition.
(A) Blocking CDH11 with sd-133 significantly reduced the proliferation of CDH11 positive MDA-MB-231 as measured by MTS assay. (B) Sd-133 did not inhibit the growth of CDH11-negative MDA-MB-435 melanoma or MCF7 breast cancer cell lines. (C) Sd-037 and Sd-133 significantly impaired MDA-MB-231 outgrowth on Matrigel™. (D) Sd-133 fails to change Matrigel™ morphology of CDH11 negative MDA-MB-435 and MCF7 cells. (E) Effect of sd-133 on anchorage independent colony growth in soft agar. (F) Colony growth at various sizes when MDA-MB-231 cells were treated with Sd-133. (G) Likely binding model of Sd-133. W-binding pocket residues are highlighted (C-atoms-white; H-bonds-red dotted lines). Residues F7, L24, S26, Y37, A75, A77, E87, S90, and F92 contribute hydrophobic interactions and a water mediated interaction with Sd-133. The hydrophobic and H-bond interaction between Sd-133 and CDH11 is similar to that of the two W as seen in (Fig. 5F). (H) Diagram of the concave surface of P1 and P2. W-binding pocket residues are highlighted (C-atoms-white; H-bonds-red dotted lines). Sd-133 is locked into the cavity with H-bond networks on the outside of the concave surface. (I) The H-bond and hydrophobic interactions of Sd-037 and (J) Sd-073 are similar to Sd-133. (K) Superimposition of Cadherin-11 inhibitors Sd-133, Sd-037 and Sd-073 (C-atoms-white) with the W of a partner EC1 monomer motif (C-atoms-green). C: control. Columns and bars show the mean and ESM respectively.