Abstract
Background
Anabolic-androgenic steroids (AASs) are abused primarily in the context of intense exercise and for the purposes of increasing muscle mass as opposed to drug-induced euphoria. AASs also modulate the HPA axis and may increase the reinforcing value of exercise through changes to stress hormone and endorphin release. To test this hypothesis, 26 adult males drawn from a larger study on AAS use completed a progressive ratio task designed to examine the reinforcing value of exercise relative to financial reinforcer.
Method
Sixteen experienced and current users (8 on-cycle, 8 off-cycle) and 10 controls matched on quantity x frequency of exercise, age, and education abstained from exercise for 24 hours prior to testing and provided 24-hour cortisol, plasma cortisol, ACTH, β-endorphin samples, and measures of mood, compulsive exercise, and body image.
Results
Between group differences indicated that on-cycle AAS users had the highest β-endorphin levels, lowest cortisol levels, higher ACTH levels than controls. Conversely, off-cycle AAS users had the highest cortisol and ACTH levels, but the lowest β-endorphin levels. Exercise value was positively correlated with β-endorphin and symptoms of AAS dependence.
Conclusion
The HPA response to AASs may explain why AASs are reinforcing in humans and exercise may play a key role in the development of AAS dependence.
Keywords: anabolic-androgenic steroids, β-endorphin, cortisol, ACTH, compulsive exercise, drug dependence
1. INTRODUCTION
Anabolic-androgenic steroid (AAS) use is a unique form of drug abuse that is not motivated by a euphoric effect, but rather the human drives to look better and achieve a competitive edge (Copeland et al., 2002). AAS use involves ingesting or injecting synthetic male hormones or their derivatives to capitalize on the role of the hypothalamic-pituitary-gondal (HPG) axis in stimulating growth and repairing the muscoskeletal system. The pharmacology of AAS use is quite complex, but all AASs share an affinity for the androgen receptor (AR) and they exert the majority of their desired effects through AR-mediated mechanisms (Kicman, 2008). These drugs are typically taken in drug “cycles” where supraphysiological doses are taken at levels that yield a 10–20 fold increase in circulating blood levels of androgenic hormones for periods of 12–24 weeks (Alen et al., 1987; Hildebrandt et al., 2007). This practice results in a positive linear dose-dependent increase in lean muscle mass when taken in the context of regular exercise (Bhasin et al, 2001; Woodhouse et al., 2004, 2003), which usually consists of a mixture of high quantity and frequency of aerobic and anaerobic activities. Although these drugs are used by an estimated 1–3% of mostly male adults in the US and 6.4% worldwide (Beaver et al., 2008; Galduroz et al., 2005; McCabe et al., 2007; Sagoe et al., 2014), very little data are available on the behavioral or endocrine effects of AAS use among experienced AAS users.
Clear models of drug dependence have been difficult to establish for AASs, in part because of the lack of drug euphoria, difficulty in identifying a pattern of drug craving, and primary role of exercise and body image disturbance in drug maintenance (Kanayama et al., 2009b). Despite the inconsistencies between AAS use and classic drug dependence, rodent research indicates that they will be self-administered (Johnson and Wood, 2001; Wood et al., 2004) even to the point of death (Peters and Wood, 2005) although they are only mildly reinforcing compared to other drugs of abuse (Wood, 2004). The absence of AASs’ effect on dopamine (DA) release in the nucleus accumbens (NAc) is likely one reason for its relative reinforcement value (Triemstra et al., 2008). More recently, rodent research has focused on alterations to the opioid system to explain AAS reinforcement (Wood, 2008). AASs administered at high doses stimulate β-endorphin release in the rodent ventral tegmental area (VTA; Johansson et al., 2000, 1997), alter the expression of kappa, delta, and mu opioid receptors in the NAc and hypothalamic nuclei (Magnusson et al., 2009), and increase the metabolism of opioid peptides (Magnusson et al., 2007). Antagonism of opioid receptors with naltrexone inhibits AAS self-administration and blocks central nervous system depression at high doses of testosterone (Peters and Wood, 2005). These data support the possibility that AASs exert some mild reinforcing effects via increases in opioid peptides centrally in the brain and possibly through analgesic effects in peripheral tissues.
There is very little investigation of these opioid mechanisms in human AAS users. Some case report data suggests high rates of prescription opiate dependence among AAS users (Wines et al., 1999). In healthy volunteers, short-term AAS administration increases plasma and CSF levels of β-endorphin (Daly et al., 2001), but little is known about the role of these β-endorphin increases in the development or maintenance of AAS dependence. To date, no study has examined the relationship between opioid levels and symptoms of AAS dependence among AAS users.
An often overlooked aspect of AAS dependence is the compulsive pattern of exercise used to bring about the desired effects of AASs. Exercise offers an interesting opportunity to explain a how AAS dependence can develop among humans despite a lack of acute euphoria. Among rodents, AASs self-administration leads to a dose-dependent increase in free-wheel running, suggesting that AASs increase the reinforcing value of exercise (Wood, 2002). Thus, AASs may elicit a high degree of drug reinforcement by hijacking the same neuorendocrine environment that makes exercise reinforcing (Hildebrandt et al., 2011). Support for this hypothesis is evident by documented overlaps in endocrine effects stimulated by AAS administration and intense exercise. Among healthy men, acute increases in testosterone are reliably found in response to exercise (Bosco et al., 2000; Gotshalk et al, 1997; Hakkinen and. Pakarinen, 1993; Kraemer, 1988; Kraemer et al, 1991, 1990; Schwab et al., 1993). Furthermore,. exercise acutely increases levels of opioids such as β-endorphin (Elias et al., 1986; Eliot et al., 1984; Farrell et al., 1987; Goldfarb et al., 1987), but may result in decreased β-endorphin response to prolonged exercise training (Lobstein and Ismail, 1989). These endorphin effects can be conceptualized as part of a larger adaptive response to the physical stress of exercise which engages the hypothalamic-pituitary-adrenal (HPA) axis to quickly mobilize energy stores to support intense physical activity. Of the glucocorticoids released during exercise, cortisol accounts for the majority of the activity. Cortisol stimulates lipolysis and decreases protein synthesis in muscle cells stimulating the release of lipids. Acutely, resistance exercise leads to increases in cortisol and ACTH (Hakkinen et al., 1988; Kraemer et al, 1996; Kraemer. et al,. 1999a, 1999b), with greater increases among experienced resistance trained men compared to endurance trained men (Tremblay et al., 2004). Among AAS users, cortisol response increases significantly less than heavy exercising controls in response to exercise (Boone et al, 1990). Thus, exercise and AAS use may become interdependent overtime because AASs facilitate an increased opioid response and decreased cortisol response to heavy exercise.
The purpose of the study was to examine differences between on-cycle AAS users, off-cycle AAS users, and a matched sample of heavy exercising controls on (a) stress hormones and β-endorphin levels, (b) the relative reinforcing value of exercise, and (c) clinical correlates of these hormonal and behavioral measures.
2. METHODS
2.1 Participants
Participants included 26 males (n=8 on-cycle steroid users; n=8 off-cycle steroid users; n=10 healthy exercising controls) from a larger ongoing longitudinal study of naturalistic AAS use designed to capture the behavioral and endocrinological changes that occur across an AAS cycle in comparison to AAS naïve heavy exercising controls matched on age, education, and amount of weekly exercise. AAS status was confirmed by urine screen (see endocrine measures below). Mean participant age was 35.61 (SD=8.81), and the sample ranged in age from 23 to 52 years old. Modal years of education for on-cycle users (13.00, SD=2.33), off-cycle users (13.00, SD =2.33) and healthy controls (12.00, SD= 2.74) with a range of 9–16 years. Participants were predominantly Caucasian (n=18, 69.2%), African-American (n=4, 15.4%), and American Indian (n=1, 3.9%), and n= 9 (34.6%) were Hispanic (n=10, 73.3%). Mean participant household income was $49, 366.67 (SD=32,514.96). The 16 AAS users in the study were experienced. They completed an average of 11.21 (SD = 8.1) AAS cycles, with an average duration of 16.1 (7.4) weeks and 15.6 (SD =12.8) weeks between cycles.
Inclusion criteria for AAS-users enrolled in the study included: (a) male, (b) at least 18 years old, (c) having completed at least one previous AAS cycle, (d) intention to go on-cycle within three months of being screened for the study, (e) planned cycle is greater than 6 weeks in duration, (h) planned cycle is less than 25 weeks in duration, and (i) planned cycle includes testosterone or one of its derivatives. Inclusion criteria for the heavy exercise control participants enrolled in the study included criteria a–b and (j) age is within 1 standard deviation of the mean of APED sample to date of consent, (k) self-reported number of years engaged in weight training is within 1 standard deviation of mean of AAS sample to date of consent, (l) self-reported frequency and duration of exercise in the past 28 days is within 1 standard deviation of mean of AAS sample to date of consent, (m) self-reported education is within 1 standard deviation of mean of AAS sample to date of consent, and (n) self-reports never having used an appearance and performance enhancing drug. Exclusion criteria for AAS-users included: (a) evidence of psychotic symptoms as assessed with the SCID I Psychotic Disorders Screening Interview (First, et al., 2007), (b) planned cycle includes a thyroid hormone, stimulant, or metabolic enhancer that is a controlled substance (i.e., illegal without a prescription), (c) current suicidality/homicidality, (d) current non-APED drug dependence assessed by SCID I at baseline interview, (e) currently “on-cycle” (i.e. actively taking APEDs) assessed by urine screen and self-reports, and (f) previous screening for the study. Exclusion criteria for control participants included criteria a, and c–f.
2.2 Procedures
2.2.1 Progressive Ratio Task
We adapted a behavioral measure of the reinforcing value of exercise from previous research on physical activity among women with anorexia nervosa (see (Klein et al, 2009; Schebendach et al., 2007). Participation for their current study involved the. completion of a specific progressive ratio work for exercise (WFE) task (see measures below) at one of the visits scheduled for the larger study. As part of their participation in that study, participants completed an extensive battery of psychiatric and behavioral measures. On the day of the WFE task, participants were asked to arrive in exercise clothing and to abstain from exercise for the previous 24-hours. All participants reported adherence to this protocol.
Testing occurred in the office of a small gym and participants were monitored by a research coordinator during completion of the task via a window. To keep consistent with existing uses of the WFE task, participants were given access to a treadmill for their receipt of their exercise payout. Alternatively, they were paid by check for the financial reinforcer. Participants were shown the treadmill before testing. Participants were allowed to exercise at their own choice of intensity. Heart rate was monitored throughout the exercise and participants were given five min to cool down on the treadmill after completing their allotted exercise. Questionnaires (see measures below) were administered immediately prior to the WFE task, immediately after the WFE task, and upon receipt of their reinforcer (after receiving check or completing exercise).
During the WFE session, an individual worked for all or part of a maximum amount of time (30 min) of exercise, or for all or part of a maximum amount of cash ($30 US). Work consisted of finger presses on a computer keyboard. Each PR session consisted of 10 trials, with 1/10th the maximum amount of exercise time or cash earned at each trial.
To begin a WFE task session, the participant selected a computer screen icon that represented the reinforcer of his choice, opportunity to exercise, or cash. Once this was selected, the participant was required to continue to work for that reinforcer until that trial was completed (or until he stopped working for the session). After completion of a trial, each participant had 8 s to decide which reinforcer he would work for at the next trial. Selection occurred only at the beginning of a trial but not within a trial (i.e., after he had pressed the keypad multiple times). The work required in the first trial for each reinforcer was 50 presses; however, the work requirement increased each subsequent trial that reinforcer was selected. At the first trial, a given reinforcer was chosen, 50 presses were required. The second time it was selected, 250 presses were required, and then 450; 650; 850; 1,050; 1,250; 1,450; 1,650; and 1,850 presses were required to complete the trial. If the participant selected the alternative reinforcer, it would require 50 button presses, then 250, 450, etc. Thus, the work requirement for each reinforcer increased independently during the session. To earn the maximum amount of exercise or cash, the participant had to perform 9,500 keyboard finger presses within the 40-min period. Fewer presses were required if choices were distributed between the two reinforcers. Participants were informed before beginning the experiment about these procedures. At the end of each trial, an icon representing the earned reinforcer was displayed on the right side of the computer screen. At the end of the 40-min session, total earnings were displayed.
2.2.2 WFE breakpoint
The largest completed number of button presses (i.e., trial) for a given reinforcer, termed the PR breakpoint, is thought to provide a measure of the reinforcing efficacy of that object or activity. A larger PR breakpoint indicates a more potent reinforcer.
2.3 Measures
2.3.1 Behavioral and Self-Report Measures
As part of the larger study, participants completed the Appearance and Performance Enhancing Drug Use Schedule (APEDUS) which is a semi-structured interview that includes comprehensive assessment of the major drug use, attitudinal, and behavioral features of AAS dependence. For this study, background and demographic information were gathered from this measure and the Compulsive Exercise subscale (CES) was used to examine correlations between WFE breakpoint and hormone levels. The APEDUS has been found to have excellent test-retest reliability (r = .83–1.0), inter-rater reliability (ICC = .81–1.0), and internal consistency for the CES (α =.88) in AAS users (Hildebrandt et al., 2011). Characteristics of typical AAS use were assessed from the Usual APED Use module of the APEDUS.
During the WFE task, participants completed the Muscle Dysmorphic Disorder Inventory (MDDI; Hildebrandt et al., 2004), which is a 13-item measure of body image disturbance derived from the diagnostic criteria for muscle dysmorphia. It has been validated in weightlifting men and used to study body image disturbance in AAS users (Hildebrandt et al., 2010). Participants also completed the Profile of Mood States (POMS; McNair et al., 1992) three times during the WFE task. The POMS has five subscales (Fatigue, Anger, Tension, Depression, Vigor, and Confusion) with higher scores reflecting higher levels of that mood state at the time of the measure.
All participants completed the POMS questionnaire at two time points (pre-WFE task and post-WFE task), and participants who earned exercise time completed it at an additional third time point (post-exercise). Participants also completed the MDDI once before beginning the task.
2.3.2 Endocrine and AAS measures
Hormone measures included 24-hour urine cortisol RIA (DiaSorin Inc; Stillwater, MN; sensitivity = 2.5 ng/mL, intra-assay CV% = 3.3%, inter-assay CV% = 10.0), plasma cortisol RIA (DiaSorin Inc; Stillwater, MN; sensitivity = 0.21 μg/dL, intra-assay CV% = 2.3%, inter-assay CV% = 9.9), plasma β-endorphin RIA (ALPCO Diagnostics; Salem, NH, sensitivity = 3.0 pmol/L, intra-assay CV% = 7.1%, inter-assay CV% = 8.2), and plasma adrenocorticotropin hormone (ACTH) ELSIA levels (ALPCO Diagnostics; Salem, NH, sensitivity = .22 pg/mL, intra-assay CV% = 3.7%, inter-assay CV% = 6.0). All assays were completed in the clinical laboratory at the James J. Peters Veterans Affairs Medical Center in the Bronx, NY.
Cycle status was confirmed by urine screen. We collected 50 mL urine sample from each participant stored at −8 °C until shipped to Antidoping Research, Inc. Testing was performed using in-house analytical methods designed for sports doping control purposes and validated to the standards of ISO/IEC 17025:2005. Solid phase extractions and liquid-liquid extractions were employed depending on the target compounds. Analysis is performed by gas chromatography/mass spectrometry (GCMS) and/or liquid chromatography/mass spectrometry (LCMS). Primary instruments used are an Agilent 5890 GCMS and a Qtrap 4000 LCMS. Detection limits for the majority of compounds in the screen is 2 ng/g including prescription opiates an illicit drugs of abuse.
2.4 Statistical Analyses
Generalized linear models (GLMs) were used to examine the effect of group (AAS-ON, AAS-OFF, Controls) on outcomes. For non-normal data, the appropriate link function was chosen based on comparison of different models within specified family (Gaussian, negative binomial, etc.) for goodness of fit using Akiake Information Criterion (AIC). Age, education, and annual household income were tested as covariates to control for outside sources of variation in the dependent variables and time of assay collection as a nuisance variable for endocrine outcomes. Linear mixed effects models were used to evaluate change in mood over the WFE procedure. Spearman rank correlations were used to describe relationships between WFE and clinical variables associated with AAS dependence. Similar correlations were calculated with hormone values. Alpha levels were set at .05 for all significance tests.
3. RESULTS
3.1 Exercise Breakpoint and Activity
There were no significant differences between groups on Age, Annual household income, or education, so these variables were not included as covariates the GLMs. Table 1 summarizes the between group differences in WFE breakpoint, Money breakpoint, and exercise measures. Both breakpoints were negatively skewed and leptokurtotic, consistent with count data. The WFE breakpoint for on-cycle users was significantly higher than control subjects (β = 1.81, SE = .47, p < .001) and off-cycle users (β = .68, SE = .34, p < .05). Off-cycle users’ WFE breakpoint was significantly higher than controls (β = 1.12, SE = .52, p < .05). On-Cycle users has significant lower Money breakpoints than healthy controls (β = 1.83, SE = .59, p < .01), but not off-cycle users (β=0.81, SE=.59, p = .17). Off-cycle users did not significantly differ from healthy controls on Money breakpoint (β = 1.00, SE = .61, p < .10). Thus, the data indicated that on-cycle AAS users found exercise to be more reinforcing and money less reinforcing than heavy exercising controls. There were no significant differences between groups in degree of compulsive exercise or quantity and frequency of exercise over the past month.
Table 1.
Between Group Differences in Exercise Quality, Quantity, and Reinforcement Level
| AAS-Off (n = 8) | AAS-On (n =8) | Heavy Exercise Control (n = 10) | F or Wald Statistic | p | Effect Size | |
|---|---|---|---|---|---|---|
| WFE Breakpoint-Exercisea | 400. 00 (387.30) | 787.50 (753.44)a | 130.00 (289.82) | 14.36 | <.001 | |
| WFE Breakpoint-Moneyb | 1350.00 (451.66) | 912.50 (669.09) | 1690 (350.23) | 9.45 | .01 | |
| Compulsive Exercise | 3.32 (0.95) | 3.46 (1.16) | 2.51 (1.03) | 1.20 | .34 | .17 |
| Quantity x Frequency of exercise | 18.00 (7.70) | 18.75 (8.20)c | 14.80 (7.85) | 0.78 | 0.49 | .04 |
Note. WFE = work for exercise. M (SD) reported for each group.
Wald statistic reported for negative binomial model for count data.
Cumulative probit model Wald statistic reported.
One statistical outlier reporting 64 hrs of exercise in the past month removed from model. Quantity x Frequency of exercise measured in hours spent exercising per month.
3.2 Endocrine Differences
Figure 2 summarizes the between group differences in plasma β-endorphin level. On-cycle AAS users had significantly higher plasma levels than either off-cycle or heavy exercising controls. An inverse Guassian model was fit to the data and provided lowest AIC values. The model fit was acceptable (scaled deviance χ2(3) = 31.288, p < .01) and examination of residual plots supported model selection. On-cycle users had significantly greater β-endorphin (β = 3.66, SE = 1.72, p < .05) levels than controls and Off-cycle users (β = 4.84, SE = 1.71, p < .01). However, Off-cycle users did not significantly differ from controls (β = −1.81, SE = 1.19, p = .32). A reverse pattern was evident for both plasma and 24 hour urinary cortisol levels (see Figure 3a–b). Generalized linear model using a Gaussian distribution and identity link for plasma cortisol provided lowest AIC for plasma cortisol. The model indicated that On-Cycle users had lower cortisol levels than Off-cycle users (β = −5.12, SE = 2.01, p < .01), but not controls (β = −2.11, SE = 1.76, p = .23). Off-cycle users did not significantly differ from controls (β = 3.01, SE = 1.97, p = .13). For urinary cortisol, the inverse Gaussian model provided acceptable fit (scaled deviance χ2(3) = 21.96, p < .01) and lowest AIC among GLMs within the Gaussian family. On-cycle users produced significantly less 24-hr urinary cortisol than Off-cycle users (β = −21.87, SE = 10.35, p < .05) and controls (β = −21.01, SE = 10.35, p < .05). Off-cycle users and controls did not significantly differ in urinary cortisol levels (β = 0.86, SE = 12.14, p = .94). Figure 4 summarizes between group differences in plasma ACTH. A GLM with Gaussian distribution and identity link provided best fit. On-cycle users had significantly greater ACTH levels than controls (β = 6.89, SE = 3.26, p = .05), but not off-cycle users (β = 2.58, SE = 3.58, p = .42). However, Off-cycle users also had higher levels of ACTH than controls (β = 9.46, SE = 3.50, p < .01).
Figure 2.
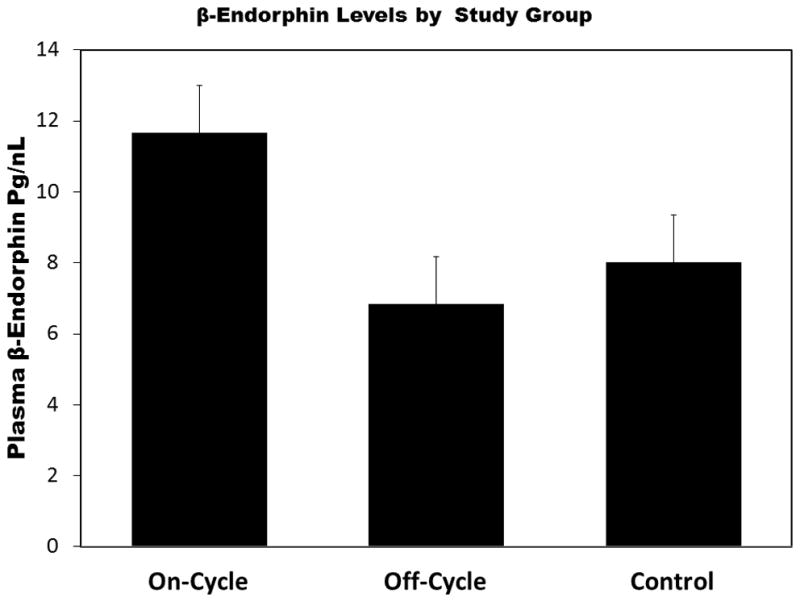
The On-cycle anabolic-androgenic steroid (AAS) users who were on-cycle had significantly higher levels of β-endorphin than off-cycle AAS users or heavy exercising controls. Wald χ2 =9.82, p < .001 for AAS group on β-endorphin. Error bars are standard error of the mean. *p < .05. **p < .01.
Figure 3.
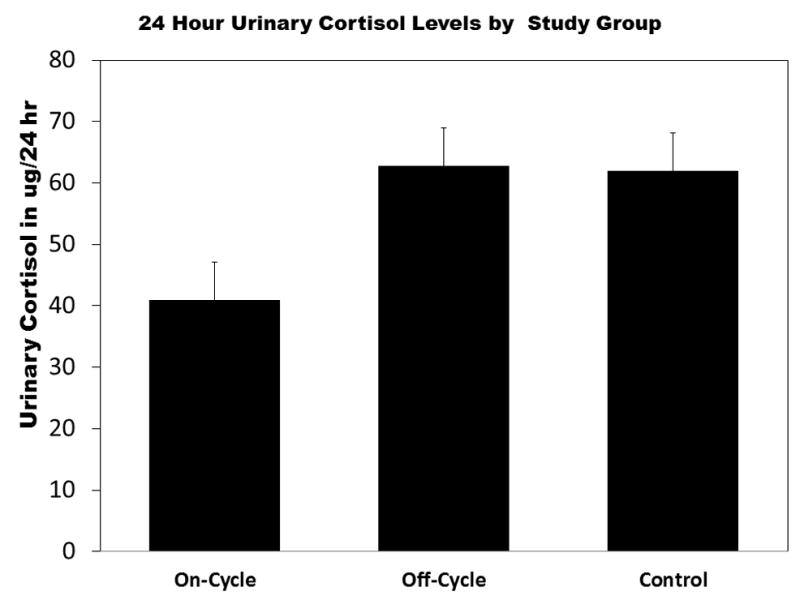
(a) The On-cycle anabolic-androgenic steroid (AAS) users had significantly higher lower plasma and 24-hr cortisol levels than off-cycle AAS users or heavy exercising controls (HECs). Likelihoood ratio (LR) χ2 =5.99, p < .05 for AAS group on plasma cortisol. (b) On-cycle AAS using group had significantly lower 24-hour urinary cortisol than (HECs) and the Off-cycle using group. LR χ2 =6.79, p < .05 for effect of APED group on 24-hour urinary cortisol. Error bars represent standard errors of the mean. *p < .05. **p < .01.
Figure 4.
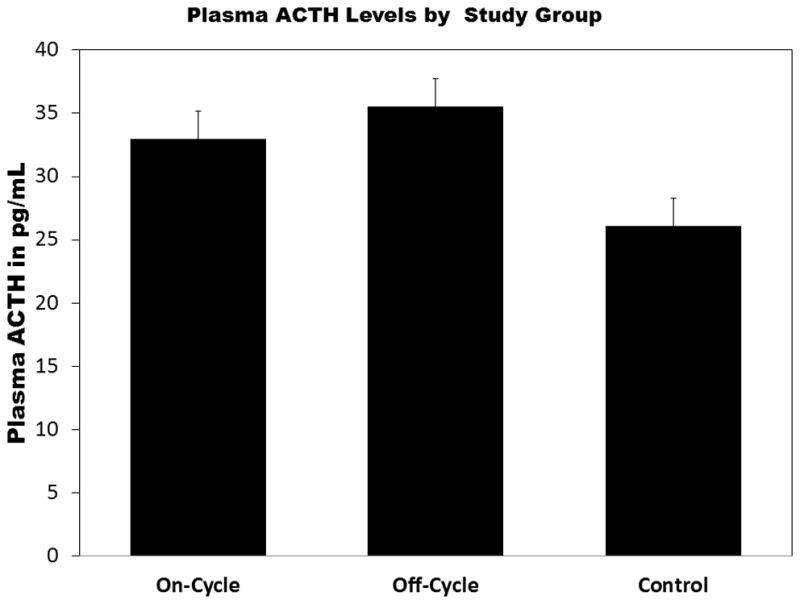
Both anabolic-androgenic steroid (AAS) using groups had significantly higher adrenocorticotropic hormone (ACTH) levels than heavy exercising controls. Wald χ2 = 8.37, p < .01 for effect of study group on ACTH. Error bars are standard errors of the mean. *p < .05. **p < .01.
3.3 Correlates of Exercise Breakpoint
Table 2 summarizes linear mixed effect models examining the change in mood among those who chose exercise and the prediction of WFE breakpoint on changes in POMS among those who earned at least 1 trial of exercise (n = 13). Figure 5 summarizes the change over time for POMS subscales and summarizes the effects on negative moods decreased over the course of the procedure and vigor did not change. Only POMS depression subscale scores demonstrated a significant study group X time interaction. Figure 6 displays interaction effect indicating that Off-cycle users, controlling for amount of exercise earned, experienced a greater decrease in depressive scores than exercising controls. The same pattern was true for On-Cycle users, but the effect was not significant. Across POMS subscales, WFE breakpoint was not a significant predictor of mood or change in mood over time.
Table 2.
Parameter Estimates for Linear Mixed Effects Models of Mood Changes Across Exercise Exposure (n = 13)
| Fatigue | Vigor | Depression | Anger | Confusion | Tense | |
|---|---|---|---|---|---|---|
| Intercept | 10.31 (3.16)** | 2.52(0.26)** | 4.65(1.55)** | 4.85(1.90)* | 4.38(1.59)* | 4.26(1.57)** |
| On-Cycle | 3.35(2.17) | −0.44(0.55) | −1.31(2.04) | −0.12(2.45) | −1.91(2.20) | 1.43(3.21) |
| Off-Cycle | 3.01(1.62) | −0.04(0.44) | 0.29(1.17) | −1.44(1.95) | −2.97(1.175) | −2.19(2.57) |
| Time | −2.14(0.40)** | 0.01(0.11) | −0.79(0.31)* | −0.81(0.39)* | −0.36(0.54) | −1.33(0.56)* |
| Exercise BP | −0.84 (0.88) | 0.25(0.18) | −0.05(0.07) | −.10(0.09) | 0.05(0.07) | 0.06(0.05) |
| On-cycle X Time | −0.64(0.84) | −0.05(0.26) | −0.54(0.65) | 0.75(0.75) | −0.39(0.79) | 0.83(1.36) |
| Off-cycle X Time | −1.02(0.66) | −0.02(0.18) | 0.59(0.51) | 1.38(0.59)* | −0.40(0.62) | 0.08(1.08) |
Note. Heavy Exercise Controls used as reference group. Log10 of Exercise Breakpoint used as predictor.
Figure 5.
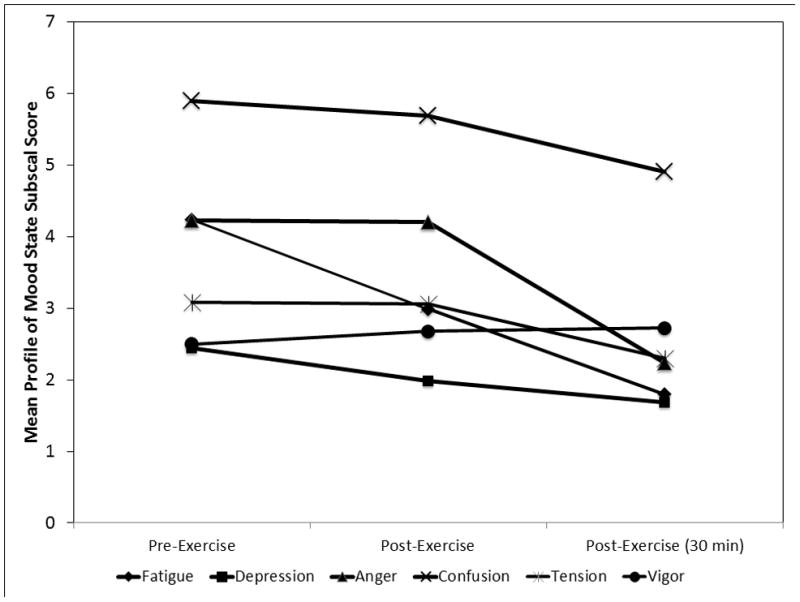
The change in specific mood states during utilization of earned treadmill time as a function of exercise in On-cycle (n = 6), Off-cycle (n = 5), and heavy exercising controls (n =2). Time points represent pre-exercise, immediately post-exercise, and 30 min post-exercise. Error pars are standard errors of the mean.
Figure 6.
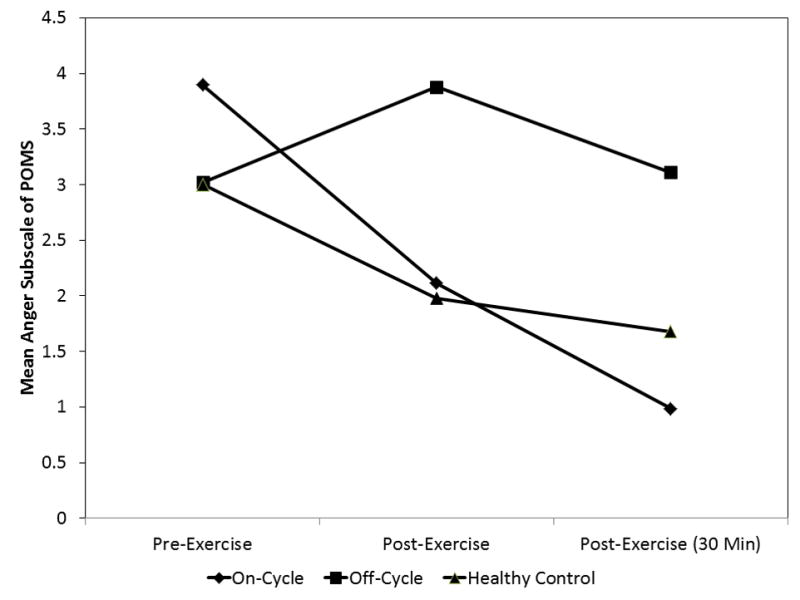
The Group X Time interaction on Profile of Mood States Anger subscale scores among On-cycle (n = 6), Off-cycle (n = 5), and heavy exercising controls (n =2). The effect indicates that Off-cycle users experienced a significantly greater decrease in anger from exercise over time, controlling for total exercise earned during work for exercise task. Time points represent pre-exercise, immediately post-exercise, and 30 min post-exercise.
Table 4 summarizes the relationship between endocrine measures and symptoms of AAS dependence. The results suggest a moderate to strong relationship between dependence symptoms and β-endorphin levels. Participants’ β-endorphin levels were negatively correlated with symptoms of dependence and with the amount and quality of exercise. Plasma ACTH levels were not significantly correlated with any of the AAS dependence symptoms, but 24 hour urinary cortisol was negatively associated with cycle length, suggesting longer cycles are associated with less cortisol production. β-endorphin level was significantly positively correlated with WFE breakpoint (r = .66, p < .01), but negatively correlated with 24-hr urinary cortisol (r = −.59, p < .05). There was no evidence of a significant relationship between WFE breakpoint and ACTH level (r = −.13, p = .66).
WFE was significantly correlated with number of AAS cycles (r =.77, p < .01), typical duration of AAS cycle (r = .59, p < .05), and the weeks between cycles (r = −.51, p < .05). However, there was no evidence that MDDI global score (r = −.07, p = .81) was significantly correlated with WFE.
4. DISCUSSION
The data from this study indicate that AAS users find exercise to be a more potent reinforcer than small amounts of money and more reinforcing than those with similarly high levels of exercise. The strength of this reinforcement is explained by an endocrine environment where β-endorphin and ACTH levels are elevated and cortisol levels are lower relative to heavy exercising controls. This profile may be an amplification of the adaptive increase in androgens observed in response to competition (Zilioli and Watson, 2013).
The role of exercise and the HPA axis in the development of AAS dependence has been theorized to involve priming of the natural allostatic response to exercise (Hildebrandt et al., 2011). Thus, AAS may enhance the reinforcing value of exercise via endorphin release or by capping the HPA response to exercise. These endocrine effects could lead to compulsive drug use in the absence of drug-induced euphoria because AASs theoretically act as a vehicle for heightened exercise reward. As demonstrated by Wood (2002), high doses of AASs also lead to a significant increase in exercise among rodents. This same relationship was evident in the present study, suggesting that opioids may increase the reinforcing value of exercise and contribute to AAS dependence. The dissociation of cortisol and β-endorphin from ACTH, however, suggest that AASs are working indirectly on processing of these hormones.
The current study did not allow for inferences about the group differences in acute stress or endorphin response to exercise, which will be important next steps in this line of research. There was some evidence that mood improved among those who completed the exercise task to a greater degree among AAS users. These changes support the possibility that those who find exercise more reinforcing have a greater mood change in response to exercise. It will be important to test whether this change is moderated by AAS use as the theory proposed by Hildebrandt et al. (2011) hypothesizes that AASs alter the acute response to exercise as well as the general hormonal environment that supports dependence on exercise and AASs.
The dissociation of ACTH from β-endorphin levels in off-cycle AAS users is also interesting because they are both derived from proopiomelanocortin (POMC). It is possible that the absence of AASs in a body adapted to high doses of androgens also results in lower levels of the enzyme production necessary to produce β-endorphin. The mechanisms of these potential AAS effects on POMC processing are unknown. There is accumulating evidence that androgens may “cap” the HPA axis response to certain stressors through active androgen metabolites including 5alpha-androstane-3beta,17beta-diol, which has affinity for estrogen receptor-β in the rat paraventricular nucleus (PVN; (Handa et al., 2009) and through direct conversion of androgens into estrogens by aromatization (Bingham et al., 2010). Thus, the increased HPA activity in the off-cycle AAS users may be due to lower levels of these androgen metabolites than available in on-cycle AAS users. Opioids also play a primary inhibitory role in the corticosterone releasing factor (CRF) neurons in the PVN, which regulate ACTH and thus cortisol release. Recent PET data suggest that high binding potential for opioid-mu receptor may lead to lower inhibitory tone and yield higher HPA activity (Wand et al., 2011). Thus, expression of the opioid-mu receptor may be a source of individual variability in the HPA response to AAS use.
The results of the current study provide continued support for the role of compulsive exercise in AAS dependence and its possible incorporation into addictive model of AAS use (Hildebrandt et al., 2011; Kanayama et al., 2009a). The fact that AASs increase lean muscle mass (Bhasin et al., 2001) and may also enhance mood and the reinforcing value of behaviors such as exercise via effects on the HPA axis suggest a powerful environment for maintaining drug use. Unfortunately, longitudinal data are unavailable to examine how these changes to the HPA axis may affect long-term psychiatric outcomes of this drug use (Kanayama et al., 2008) and the mechanisms that cause these changes remain understudied.
Figure 1.
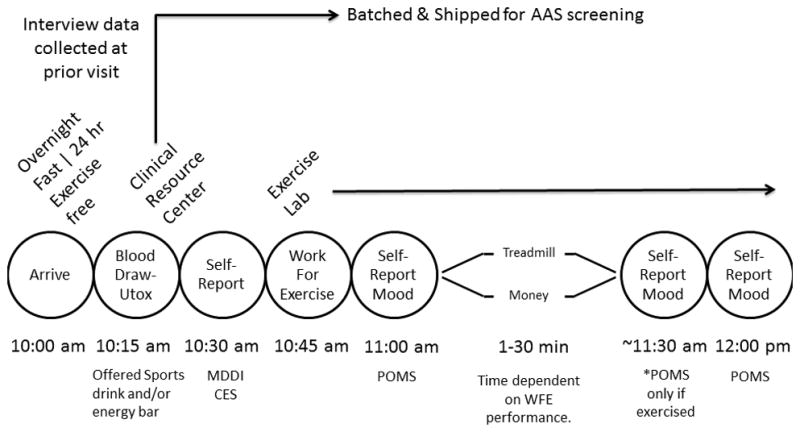
The procedural timeline involved participants abstaining from exercise for 24 hours and executing an overnight fast prior to blood draw and urine collection. Participants were offered a sports drink and energy bar after blood draw to eat when completing initial self-report measures. They were then escorted to the exercise lab to complete the work for exercise task and they received all rewards (money and/or exercise) after task. Self-reported mood was assessed pre, post, and 30 min after exercise period. POMS = profile of mood states. CES = compulsive exercise scale. MDDI = muscle dysmorphic disorder inventory.
Table 3.
Correlations between Stress Hormones, β-Endorphins, and Clinical Symptoms of AAS Dependence
| AAS Dependene | Number of AAS Cycles | Duration of AAS Cycle | Weeks Between Cycles | Compulsive Exercise | Q x F Exercise | MDDI Global Score | |
|---|---|---|---|---|---|---|---|
| β-endorphin | .56* | .42** | .57* | −.71** | .54* | .23 | .09 |
| ACTH | .33 | .28 | .27 | .11 | .18 | .04 | .18 |
| 24 hr Cortisol | .02 | .32 | −.57* | −.26 | −.20 | .16 | .09 |
| Plasma Cortisol | .36 | .16 | .04 | −.22 | −.18 | −.05 | −.14 |
Note. Log transformations used for non-normal distributions of hormones and AAS symptom counts. AAS = Anabolic-androgenic steroid. ACTH = adrenocorticotropic hormone. Q x F = quantity x frequency of exercise over last 28 days.
p < .05.
p < .01.
Spearman correlations reported.
Acknowledgments
Role of Funding Source
Funds for this study were provided by NIDA K23 grant DA024043 awarded to Dr. Hildebrandt. NIDA had no influence over the study design, execution of the project, interpretation or results, or manuscript preparation.
We would like to thank the staff of the Respiratory Therapy department at Mount Sinai for allowing us access to their gym facilities in which we conducted the Work for Exercise task. We would also like to thank the Mount Sinai Clinical Research Unit for conducting the blood draws as required by the study protocol.
Footnotes
Contributors
Drs. Hildebrandt, Klein, Pfaff, and Yehuda were responsible for the conception of the study and primary interpretation of the study findings. Dr Hildebrandt was responsible for the first draft of the manuscript and conducting the primary statistical analyses. Ms. Varangis and Ms. Shope were responsible for executing study procedures and contributed to the statistical analyses. All authors contributed to the manuscript and approved the final version.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alen M, Rahkila P, Reinila M, Vihko R. Androgenic-anabolic steroid effects on serum thyroid, pituitary and steroid hormones in athletes. Am J Sports Med. 1987;15:357–361. doi: 10.1177/036354658701500411. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Vaughn MG, Delisi M, Wright JP. Anabolic-androgenic steroid use and involvement in violent behavior in a nationally representative sample of young adult males in the United States. Am J Public Health. 2008;98:2185–2187. doi: 10.2105/AJPH.2008.137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Bingham B, Gray M, Sun T, Viau V. Postnatal blockade of androgen receptors or aromatase impair the expression of stress hypothalamic-pituitary-adrenal axis habituation in adult male rats. Psychoneuroendocrinology. 2010;36:249–257. doi: 10.1016/j.psyneuen.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Boone JB, Jr, Lambert CP, Flynn MG, Michaud TJ, Rodriguez-Zayas JA, Andres FF. Resistance exercise effects on plasma cortisol, testosterone and creatine kinase activity in anabolic-androgenic steroid users. Int J Sports Med. 1990;11:293–297. doi: 10.1055/s-2007-1024810. [DOI] [PubMed] [Google Scholar]
- Bosco C, Colli R, Bonomi R, von Duvillard SP, Viru A. Monitoring strength training: neuromuscular and hormonal profile. Med Sci Sports Exerc. 2000;32:202–208. doi: 10.1097/00005768-200001000-00030. [DOI] [PubMed] [Google Scholar]
- Cohen J. Stastical Power for Analysis in the Behavioral Sciences. 2. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Copeland J, Peters R, Dillon P. Anabolic-androgenic steroid use disorders among a sample of Australian competitive and recreational users. Drug Alcohol Depend. 2000;60:91–96. doi: 10.1016/s0376-8716(99)00141-6. [DOI] [PubMed] [Google Scholar]
- Daly RC, Su TP, Schmidt PJ, Pagliaro M, Pickar D, Rubinow DR. Neuroendocrine and behavioral effects of high-dose anabolic steroid administration in male normal volunteers. Psychoneuroendocrinology. 2003;28:317–331. doi: 10.1016/s0306-4530(02)00025-2. [DOI] [PubMed] [Google Scholar]
- Daly RC, Su TP, Schmidt PJ, Pickar D, Murphy DL, Rubinow DR. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Arch Gen Psychiatry. 2001;58:172–177. doi: 10.1001/archpsyc.58.2.172. [DOI] [PubMed] [Google Scholar]
- Elias AN, Iyer K, Pandian MR, Weathersbee P, Stone S, Tobis J. Beta-endorphin/beta-lipotropin release and gonadotropin secretion after acute exercise in normal males. J Appl Physiol. 1986;61:2045–2049. doi: 10.1152/jappl.1986.61.6.2045. [DOI] [PubMed] [Google Scholar]
- Eliot DL, Goldberg L, Watts WJ. Resistance exercise and plasma beta-endorphin/beta-lipotrophin immunoreactivity. Life Sci. 1984;34:515–518. doi: 10.1016/0024-3205(84)90483-1. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Kjaer M, Bach FW, Galbo H. Beta-endorphin and adrenocorticotropin response to supramaximal treadmill exercise in trained and untrained males. Acta Physiol Scand. 1987;130:619–625. doi: 10.1111/j.1748-1716.1987.tb08184.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2007. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (Vol. November, 2002) [Google Scholar]
- Galduroz JC, Noto AR, Nappo SA, Carlini EA. Household survey on drug abuse in Brazil: study involving the 107 major cities of the country--2001. Addict Behav. 2005;30:545–556. doi: 10.1016/j.addbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Goldfarb AH, Hatfield BD, Sforzo GA, Flynn MG. Serum beta-endorphin levels during a graded exercise test to exhaustion. Med Sci Sports Exerc. 1987;19:78–82. [PubMed] [Google Scholar]
- Gotshalk LA, Loebel CC, Nindl BC, Putukian M, Sebastianelli WJ, Newton RU, Hakkinen K, Kraemer WJ. Hormonal responses of multiset versus single-set heavy-resistance exercise protocols. Can J Appl Physiol. 1997;22:244–255. doi: 10.1139/h97-016. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Pakarinen A. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol. 1993;74:882–887. doi: 10.1152/jappl.1993.74.2.882. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Pakarinen A, Alen M, Kauhanen H, Komi PV. Neuromuscular and hormonal adaptations in athletes to strength training in two years. J Appl Physiol. 1988;65:2406–2412. doi: 10.1152/jappl.1988.65.6.2406. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Alfano L, Langenbucher J. Body image disturbance among 1000 appearance and performance enhancing drug users. J Psychiat Res. 2010;44:841–846. doi: 10.1016/j.jpsychires.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Lai JK, Langenbucher JW, Schneider M, Yehuda R, Pfaff DW. The diagnostic dilemma of pathological appearance and performance enhancing drug use. Drug Alcohol Depend. 2011;114:1–11. doi: 10.1016/j.drugalcdep.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher J, Lai JK, Loeb KL, Hollander E. The development and validation of the Appearance and Performance Enhancing Drug Use Schedule. Addict Behav. 2011;36:949–958. doi: 10.1016/j.addbeh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher J, Schlundt DG. Muscularity concerns among men: development of attitudinal and perceptual measures. Body Image. 2004;1:169–181. doi: 10.1016/j.bodyim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher JW, Carr SJ, Sanjuan P. Modeling population heterogeneity in appearance- and performance-enhancing drug (APED) use: applications of mixture modeling in 400 regular APED users. J Abnorm Psychol. 2007;116:717–733. doi: 10.1037/0021-843X.116.4.717. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Yehuda R, Alfano L. What can allostasis tell us about anabolic-androgenic steroid addiction? Dev Psychopathol. 2011;23:907–918. doi: 10.1017/S0954579411000393. [DOI] [PubMed] [Google Scholar]
- Johansson P, Hallberg M, Kindlundh A, Nyberg F. The effect on opioid peptides in the rat brain, after chronic treatment with the anabolic androgenic steroid, nandrolone decanoate. Brain Res Bull. 2000;51:413–418. doi: 10.1016/s0361-9230(99)00263-4. [DOI] [PubMed] [Google Scholar]
- Johansson P, Ray A, Zhou Q, Huang W, Karlsson K, Nyberg F. Anabolic androgenic steroids increase beta-endorphin levels in the ventral tegmental area in the male rat brain. Neurosci Res. 1997;27:185–189. doi: 10.1016/s0168-0102(96)01141-8. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Wood RI. Oral testosterone self-administration in male hamsters. Neuroendocrinology. 2001;73:285–292. doi: 10.1159/000054645. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG. Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009a;104:1966–1978. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG. Issues for DSM-V: clarifying the diagnostic criteria for anabolic-androgenic steroid dependence. Am J Psych. 2009b;166:642–644. doi: 10.1176/appi.ajp.2009.08111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG. Long-term psychiatric and medical consequences of anabolic-androgenic steroid use: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154:502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DA, Schebendach JE, Gershkovich M, Bodell LP, Foltin RW, Walsh BT. Behavioral assessment of the reinforcing effect of exercise in women with anorexia nervosa: further paradigm development and data. Int J Eat Disord. 2010;43:611–618. doi: 10.1002/eat.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer WJ. Endocrine responses to resistance exercise. Med Sci Sports Exerc. 1988;20(Suppl):S152–157. doi: 10.1249/00005768-198810001-00011. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Clemson A, Triplett NT, Bush JA, Newton RU, Lynch JM. The effects of plasma cortisol elevation on total and differential leukocyte counts in response to heavy-resistance exercise. Eur J Appl Physiol Occup Physiol. 1996;73:93–97. doi: 10.1007/BF00262815. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Fleck SJ, Maresh CM, Ratamess NA, Gordon SE, Goetz KL, Harman EA, Frykman PN, Volek JS, Mazzetti SA, Fry AC, Marchitelli LJ, Patton JF. Acute hormonal responses to a single bout of heavy resistance exercise in trained power lifters and untrained men. Can J Appl Physiol. 1999a;24:524–537. doi: 10.1139/h99-034. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Gordon SE, Fleck SJ, Marchitelli LJ, Mello R, Dziados JE, Friedl K, Harman E, Maresh C, Fry AC. Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med. 1991;12:228–235. doi: 10.1055/s-2007-1024673. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Hakkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW, Putukian M, Evans WJ. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol. 1999b;87:982–992. doi: 10.1152/jappl.1999.87.3.982. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, Mello R, Frykman P, McCurry D, Fleck SJ. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol. 1990;69:1442–1450. doi: 10.1152/jappl.1990.69.4.1442. [DOI] [PubMed] [Google Scholar]
- Lobstein DD, Ismail AH. Decreases in resting plasma beta-endorphin/-lipotropin after endurance training. Med Sci Sports Exerc. 1989;21:161–166. [PubMed] [Google Scholar]
- Magnusson K, Birgner C, Bergstrom L, Nyberg F, Hallberg M. Nandrolone decanoate administration dose-dependently affects the density of kappa opioid peptide receptors in the rat brain determined by autoradiography. Neuropeptides. 2009;43:105–111. doi: 10.1016/j.npep.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Magnusson K, Hallberg M, Bergquist J, Nyberg F. Enzymatic conversion of dynorphin A in the rat brain is affected by administration of nandrolone decanoate. Peptides. 2007;28:851–858. doi: 10.1016/j.peptides.2006.12.011. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Brower KJ, West BT, Nelson TF, Wechsler H. Trends in non-medical use of anabolic steroids by U.S. college students: results from four national surveys. Drug Alcohol Depend. 2007;90:243–251. doi: 10.1016/j.drugalcdep.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EDITS manual profile of mood states. Educational and Industrial Testing Service; San Diego, CA: 1992. [Google Scholar]
- Monaghan LF. Vocabularies of motive for illicit steroid use among bodybuilders. Soc Sci Med. 2002;55:695–708. doi: 10.1016/s0277-9536(01)00195-2. [DOI] [PubMed] [Google Scholar]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130:971–981. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014 doi: 10.1016/j.annepidem.2014.01.009. [Epub ahead of print accessed Jan 30, 2014 ]. pii: S1047-2797(14)00039-8. [DOI] [PubMed] [Google Scholar]
- Schebendach JE, Klein DA, Foltin RW, Devlin MJ, Walsh BT. Relative reinforcing value of exercise in inpatients with anorexia nervosa: model development and pilot data. Int J Eat Disord. 2007;40:446–453. doi: 10.1002/eat.20392. [DOI] [PubMed] [Google Scholar]
- Schwab R, Johnson GO, Housh TJ, Kinder JE, Weir JP. Acute effects of different intensities of weight lifting on serum testosterone. Med Sci Sports Exerc. 1993;25:1381–1385. [PubMed] [Google Scholar]
- Tremblay MS, Copeland JL, Van Helder W. Effect of training status and exercise mode on endogenous steroid hormones in men. J Appl Physiol. 2004;96:531–539. doi: 10.1152/japplphysiol.00656.2003. [DOI] [PubMed] [Google Scholar]
- Triemstra JL, Sato SM, Wood RI. Testosterone and nucleus accumbens dopamine in the male Syrian hamster. Psychoneuroendocrinology. 2008;33:386–394. doi: 10.1016/j.psyneuen.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Weerts EM, Kuwabara H, Frost JJ, Xu X, McCaul ME. Naloxone-induced cortisol predicts mu opioid receptor binding potential in specific brain regions of healthy subjects. Psychoneuroendocrinology. 2011;36:1453–1459. doi: 10.1016/j.psyneuen.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines JD, Gruber AJ, Pope HG, Lukas SE. Nalbuphine hydrochloride dependence in anabolic steroid users. Am J Addict. 1999;8:161–164. doi: 10.1080/105504999305965. [DOI] [PubMed] [Google Scholar]
- Wood RI. Oral testosterone self-administration in male hamsters: dose-response, voluntary exercise, and individual differences. Horm Behav. 2002;41:247–258. doi: 10.1006/hbeh.2002.1769. [DOI] [PubMed] [Google Scholar]
- Wood RI. Reinforcing aspects of androgens. Physiol Behav. 2004;83:279–289. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Wood RI. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol. 2008;29:490–506. doi: 10.1016/j.yfrne.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology (Berl) 2004;171:298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- Wood RI, Peters KD. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol. 2008;29:490–506. doi: 10.1016/j.yfrne.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse LJ, Gupta N, Bhasin M, Singh AB, Ross R, Phillips J, Bhasin S. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab. 2004;89:718–726. doi: 10.1210/jc.2003-031492. [DOI] [PubMed] [Google Scholar]
- Woodhouse LJ, Reisz-Porszasz S, Javanbakht M, Storer TW, Lee M, Zerounian H, Bhasin S. Development of models to predict anabolic response to testosterone administration in healthy young men. Am J Physiol Endocrinol Metab. 2003;284:E1009–1017. doi: 10.1152/ajpendo.00536.2002. [DOI] [PubMed] [Google Scholar]
- Zilioli S, Watson NV. Winning isn’t everything: mood and testosterone regulate the response to competition. PLoS One. 2013;8:e52582. doi: 10.1371/journal.pone.0052582. [DOI] [PMC free article] [PubMed] [Google Scholar]


