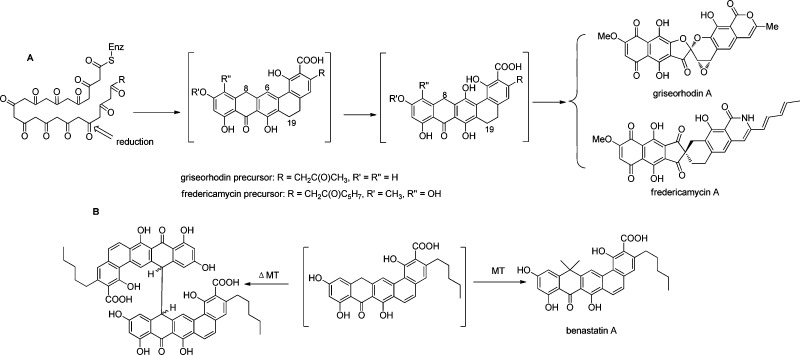
Figure 1.
Structural diversity in the griseorhodin and its close biosynthetic relatives. (A) Postulated early steps in griseorhodin and fredericamycin biosynthesis. The structures of the intermediates in brackets are supported by 1H NMR spectra and MS data. Here, we show that for the griseorhodin case the spectra represent those of a dimer. (B) Benastatin biosynthesis. A methyltransferase knockout led to accumulation of an unstable dimer, while methylation affords the natural product. The dimer in the griseorhodin pathway is similar to that of benastatin.