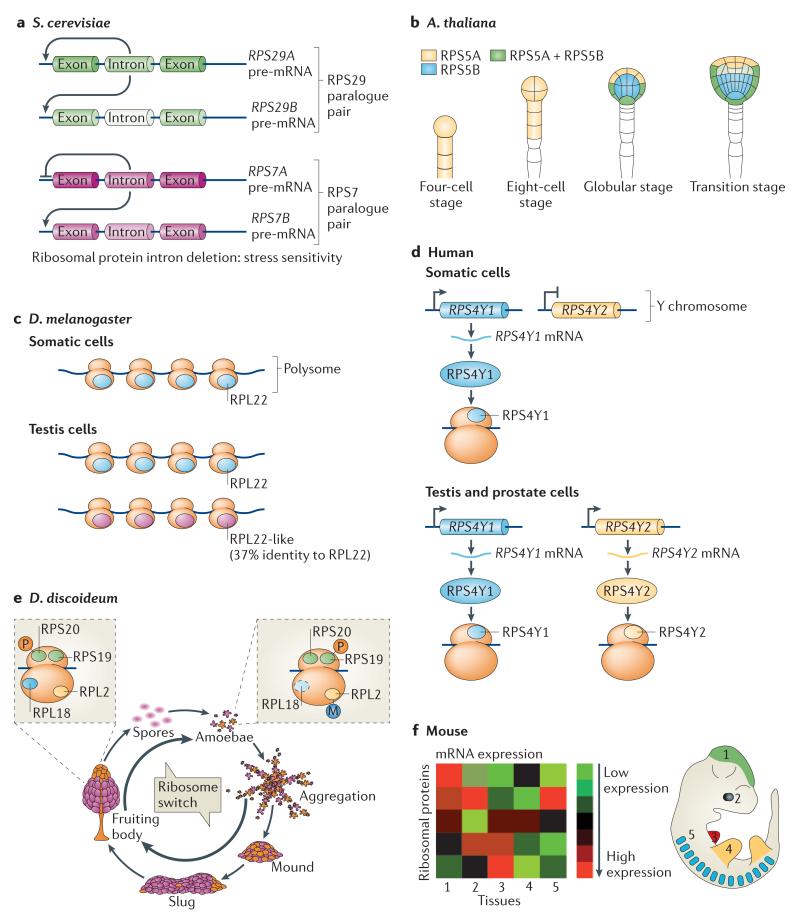
Figure 1. Heterogeneity in the ribosome at the level of ribosomal protein composition and post-translational modifications across different species.
a | Introns of genes encoding ribosomal proteins in Saccharomyces cerevisiae regulate the expression of both the intron-containing ribosomal protein genes and their paralogues, and this has important outcomes in cells: for example, increased fitness under stress. 70% of ribosomal protein paralogue pairs show differential expression. b | In plants, ribosomal protein paralogues have different functions and different expression patterns. For example, in Arabidopsis thaliana, RPS5A is expressed in rapidly dividing cells early in embryonic development, whereas RPS5B is expressed in cells undergoing differentiation. c | In Drosophila melanogaster, ribosomal protein paralogues show different expression patterns in the adult testes. For example, RPL22 is expressed ubiquitously, but RPL22-like protein levels are specifically increased in the testes. Both proteins are incorporated into translationally active ribosomes (called the polysomes). d | In humans, only some ribosomal protein paralogues have been identified; however, notable examples exist. RPS4Y1 is expressed ubiquitously, whereas RPS4Y2 is restricted to the testis and prostate. e | In the life cycle of the social amoebae Dictyostelium discoideum, ribosome switches are characterized by changes in ribosome composition at the level of ribosomal protein expression and post-translational modifications. For example, phosphorylation (P) on RPS19 and methylation (M) on RPL2 are lost as D. discoideum aggregates from a single cell amoebae to a multicellular fruiting body, while RPL20 gains phosphorylation marks. RPL18 is exclusively found in ribosomes of developing cells and not in those of the amoebae stage. f | In mice, the mRNA expression pattern of ribosomal proteins varies dramatically among tissues. This may potentially translate into unique ribosomal protein compositions of ribosomes in distinct cell types. Part a is modified, with permission, from REF. 19 © (2011) Elsevier. Part b is modified, with permission, from REF. 24 © (2001) The Company of Biologists. Part f is modified, with permission, from REF. 37 © (2011) Elsevier.