Abstract
Animal and human studies have demonstrated that early pain experiences can produce alterations in the nociceptive systems later in life including increased sensitivity to mechanical, thermal, and chemical stimuli. However, less is known about the impact of neonatal immune challenge on future responses to noxious stimuli and the reactivity of neural substrates involved in analgesia. Here we demonstrate that rats exposed to Lipopolysaccharide (LPS; 0.05 mg/kg IP, Salmonella enteritidis) during postnatal day (PND) 3 and 5 displayed enhanced formalin-induced flinching but not licking following formalin injection at PND 22. This LPS-induced hyperalgesia was accompanied by distinct recruitment of supra-spinal regions involved in analgesia as indicated by significantly attenuated Fos-protein induction in the rostral dorsal periaqueductal grey (DPAG) as well as rostral and caudal axes of the ventrolateral PAG (VLPAG). Formalin injections were associated with increased Fos-protein labelling in lateral habenula (LHb) as compared to medial habenula (MHb), however the intensity of this labelling did not differ as a result of neonatal immune challenge. These data highlight the importance of neonatal immune priming in programming inflammatory pain sensitivity later in development and highlight the PAG as a possible mediator of this process.
Introduction
Neonatal pain experiences such as hindpaw incision or inflammation are known to produce developmentally regulated changes in the nociceptive pathways, and subsequently exaggerated responses to future noxious and non-noxious stimuli [1]–[3]. Both clinical and animal studies have shown that changes in endogenous pain modulation can also occur as a consequence of neonatal inflammatory pain [4]–[6]. Although the effects of neonatal pain experiences on processing of pain later in life are well documented, the impact of neonatal exposure to mild stimuli, such as lipopolysaccharide (LPS), on subsequent inflammatory pain responses is less well understood.
Exposure to LPS during perinatal life is an established model of early life immune stress [7]–[10]. Our laboratory and others have demonstrated that neonatal LPS exposure alters immune and neuroendocrine function later in life [11]–[13]. Interestingly, we recently demonstrated that neonatal LPS exposure can also affect inflammatory pain responses, as indicated by enhanced susceptibility to formalin-induced flinching in LPS-challenged preadolescent rats [10]. We have also shown that LPS-induced behavioural hyperalgesia observed at postnatal day (PND) 22 is accompanied by increased plasma corticosterone responses and changes in the intrinsic properties of spinal dorsal horn neurons [10].
The inhibitory descending pathways travelling from the periaqueductal grey (PAG) via the dorsolateral funiculus (DLF) to the rostroventromedial medulla (RVM) and the spinal dorsal horn constitute a major anatomical circuit for the descending modulation of pain [14], [15]. This system becomes fully mature by the third week of development [16]. The PAG serves key functions in this inhibitory descending pathway including promoting analgesia [17]–[20]. For instance, formalin injection into the hindpaw of infant and adult rats is associated with Fos expression in the PAG [21], [22]. Further, PAG stimulation or an injection of morphine prior to formalin injection attenuated formalin-induced nociception [23]. Anatomically, the PAG is organized into longitudinal columns, namely dorsal PAG (DPAG), lateral PAG (LPAG) and ventrolateral PAG (VLPAG) [24]. Whereas electrical, opiate, or amino acid stimulation of the ventral PAG elicits opioid-dependent analgesia, electrical or glutamatergic stimulation of the DPAG or LPAG induces analgesia that is not blocked by opioid antagonist (e.g. naloxone) [24]–[27]. One structure that provides significant input onto the PAG to mediate antinociception is the habenula, particularly its lateral subdivision (LHb) [28], [29]. Morphological and electrophysiological studies have indicated that the habenula receives and modulates noxious inputs [30], [31]. For example, electrical stimulation or microinjection of morphine into the habenula induces analgesia in the formalin test [32], [33] and an injection of formalin into the hindpaw of rats is reported to elicit the expression of Fos-protein within the LHb [34]. Formalin injections are also associated with increased Fos-protein expression in stress-sensitive regions, including the paraventricular thalamus (PVT) [22].
Clearly, early life events are important in shaping the nociceptive circuitry. We have previously demonstrated that neonatal LPS exposure exerts developmentally regulated changes in formalin-induced behaviours, corticosterone levels, and dorsal horn neuronal properties with pronounced changes observed particularly at PND 22 [10]. In the present study, we aim to determine the supra-spinal changes associated with the neonatal immune challenge in PND 22 rats. Specifically, whether the increased flinching responses observed in preadolescent rats treated with LPS as neonates is associated with altered neuronal activity within specific subregions of the PAG, an essential substrate for opioid-induced analgesia [35], [36]. Our hypothesis is that exposure to LPS during the first postnatal week, when the descending inhibitory systems are still functionally immature [16], will alter the neuronal activity within the PAG and subsequently alter the behavioural responses to formalin injection at PND 22.
Materials and Methods
Animals and Ethics Statement
Five experimentally naïve female Wistar rats were obtained from the University of Newcastle Animal House and allowed one week acclimatisation, after which two males were introduced to their cages. The males were removed after two weeks and dams were housed individually in custom designed polycarbonate-perspex home boxes (43.5 cm×28 cm×12.5 cm; Mascot Wire Works, Sydney, Australia). Mating occurred at the University of Newcastle Psychology vivarium and resulted in a total of 68 offspring, from which a subset of males (n = 13) was selected for use in this study. A maximum of three pups per litter were assigned to each group and for each experimental condition; animals were distributed as evenly as possible from all litters used per treatment to avoid potential litter effects. Until their allocated testing day, rats were maintained in a temperature (21±1°C) and humidity (60%) controlled environment, under a 12 h/12 h light-dark cycle (light on 06∶00 h) with food (Rat and Mouse Pellets, Glen Forest, Western Australia) and water available ad libitum. All experiments were carried out in accordance with the National Health and Medical Research Council Australian Code of Practice for the care and use of animals for scientific practice. All procedures were reviewed and approved by the University of Newcastle Ethics committee (Ethics approval no. A-2010-127).
Neonatal Endotoxin Exposure
At PND 3 and 5 (birth considered as PND 1), pups were briefly removed from their home cages, weighed and administered intraperitoneally (i.p) with either LPS (from Salmonella enterica, serotype enteritidis; Sigma-Aldrich, USA, dissolved in 20 µl sterile pyrogen-free saline, 0.05 mg/kg) or an equivalent volume of sterile saline (Livingstone International, Australia). All injections were made between 9∶00 am and 10∶00 am. This model has been previously used in our [37], [38] and other laboratories [12], [39] with the dosage and timing having been demonstrated to induce a rapid sustained febrile response, but no mortality.
Formalin Testing Procedure
Unlike traditional tests of nociception such as the tail flick and hot plate tests which investigate acute pain, the formalin test is a widely accepted model of persistent pain and more closely resembles clinical cases of chronic pain [40]. Injection of formalin into the hindpaw of rodents produces a characteristic biphasic response of flinching and licking of the injected paw, with an early phase (0–5 min) and a late phase lasting up to 90 min [41], [42].
The formalin solution was prepared using Formaldehyde (36.5%–38%; Biolab Ltd, Victoria, Australia) and preservative-free saline (Sodium chloride Injection BP 0.9%, Pfizer, Australia). At PND 22, all rats (neonatal saline: n = 6; neonatal LPS: n = 7) underwent a subcutaneous injection of 1.1% formalin into the plantar surface of the left hindpaw using a 31 G needle (10 µl). The choice of formalin concentration range, volume and site of injection was based on our previous work [42]. Rats were tested in transparent Plexiglas boxes (30 cm (w)×30 cm (l)×30 cm (h)). A mirror was mounted 45° beneath the floor to allow for an unobstructed view of the paws and a camera was mounted to record the behavioural responses from the reflection of the mirror. Behavioural recording was done on a DVD recorder for one-hour post formalin injection. Flinching and licking were scored based on the method of Wheeler-Aceto and Cowan [43] and was carried out by a trained experimenter blind to treatment condition of each animal. The one-hour of behavioural recording was divided into an early phase (the first 5 min) and a late phase (10 to 60 min) during which the frequency of flinching (paw lifting or shaking) as well as the duration licking the injected paw (in seconds) was scored. PND 22 rats were tested at room temperature and at this age, an acclimation period was not required as during this period of development rats are still unable to recognize and interact with the environment [44].
No saline-injected rats were included in this study since it has been previously shown that rats subjected to a subcutaneous injection of saline into the plantar surface of the hindpaw do not display flinching or licking behaviour when tested during the first three postnatal weeks and adulthood [45]–[47]. Prior spinal cord Fos studies showed that infant rats receiving less than 20 µl saline demonstrated no Fos labelling [48].
Perfusion, Brain Collection and Immunohistochemistry
Rats were deeply anaesthetized with an overdose of Lethabarb (2 mg/kg i.p; Virbac, Pty. Ltd, Milperra, Australia) one and a half hours following the formalin test. This time point was selected as Fos-protein expression has been shown to peak at 1.5–2 hrs following stimuli exposure [49], [50]. Animals were transcardially perfused with 150 mls of normal saline followed by 500 mls of 4% paraformaldehyde (pH 9.5). Brains were removed, postfixed and cryoprotected (24 hours, at 4°C) in the same fixative solution with the addition of 15% sucrose. Brains were then stored in 15% sucrose in 0.1 M phosphate buffer (pH 7.4 at 4°C). Serial coronal sections of the rostral forebrain (40 µm) and caudal midbrain (50 µm) were cut on a freezing microtome (Leica SM 2000R, Leica Biosystems, Germany). A 1-in-4 series of brain sections from the habenula (lateral and medial sections, bregma −2.16 to −3.60) and the PVT (bregma −2.16 to −3.60), and a 1-in-5 series of the rostral (bregma −7.64), medial (bregma −8.0) and caudal (bregma −8.3) PAG [51] were processed for immunohistochemical detection of Fos-protein (72 h, 1∶10000, rabbit polyclonal, Santa Cruz Biotechnology, CA, USA) as described previously [52], [53]. Sections were then incubated in a secondary antibody biotinylated anti-rabbit (2 hours, 1∶300 donkey anti-rabbit, Jackson ImmunoResearch, PA, USA). Finally, sections were incubated in diaminobenzodine (DAB) in 2% filtered nickel sulphate (NiSO4) for 15 minutes before glucose oxidase (0.2 µL per mL of solution) was added to visualise Fos-protein.
Fos-positive cell counts were determined by creating boundaries around each brain structure. The VLPAG boundary did not include the raphe nuclei. After selecting the region of interest, a thresholding procedure was used for Fos expression. Fos counts were quantified using MetaMorph Imaging System Software (Version 7.5; MDS Analytical Technologies) under a 10x microscopic objective (Olympus CX40).
Statistical Analysis
Data analysis was carried out using the Statistical Package for the Social Sciences for Windows, version 20 (SPSS). A Linear Mixed Model (LMM) was applied to analyse the behavioural, and Fos-protein immunohistochemistry data (For more details about LMM, see [42]). For behavioural responses, the area under the curve (AUC) which was the sum of flinching or licking responses from 10 to 60 min was calculated and analysis was carried out using an ANCOVA with litter size as a covariate variable. Post-hoc analyses (two-tailed) were carried out using Least Significant Differences (LSD) tests. An alpha value of 0.05 was adopted for all tests.
Results
Formalin Responses in Preadolescent Rats: Neonatal Immune Challenge Alters Formalin-induced Nociception in both the Early and Late Phase
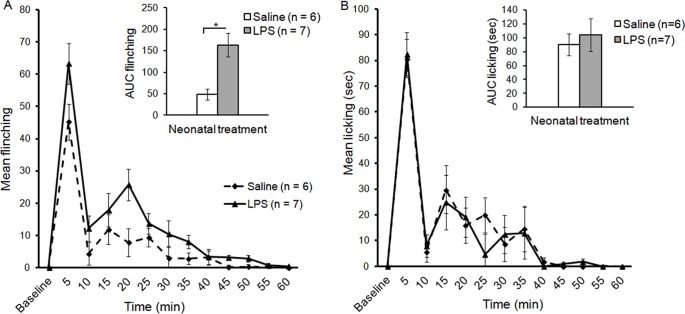
The characteristic biphasic response was observed in preadolescent rats in both treatment groups in flinching and licking of the injected paw. However, neonatal LPS-treated rats displayed higher flinching during the early and late phase. LMM analysis of flinching responses revealed a significant two way interaction of Time and Treatment [F(7,11) = 8.02, p<.01] implying that neonatal LPS treatment altered the inflammatory pain response in preadolescent rats. Pairwise comparisons revealed that LPS treated rats displayed significantly higher flinching responses during the late phase at 20 min post formalin injection when compared to their matched saline control group (p<.05; Figure 1A). Pairwise comparisons also revealed that during the early phase (first 5 min), LPS-treated rats had significantly higher flinching responses compared to saline (p<.05). Moreover, analysis of the AUC during the late phase revealed that LPS treated rats had significantly higher flinching responses compared to the saline group [F(1,11) = 5.25, p<.05; Figure 1A]. LMM analysis revealed no significant differences in licking responses between neonatally saline or LPS-treated rats in both the early and late phase of the formalin test (Figure 1B).
Figure 1. Neonatal LPS exposure enhances formalin-induced nociception in preadolescent rats.
Time course of flinching (A) and licking (B) responses following an injection of 1.1% formalin (mean ± SEM). AUC: the Area Under the Curve.
Formalin-induced Fos-protein Expression in Brain Areas Involved in Stress and Analgesia
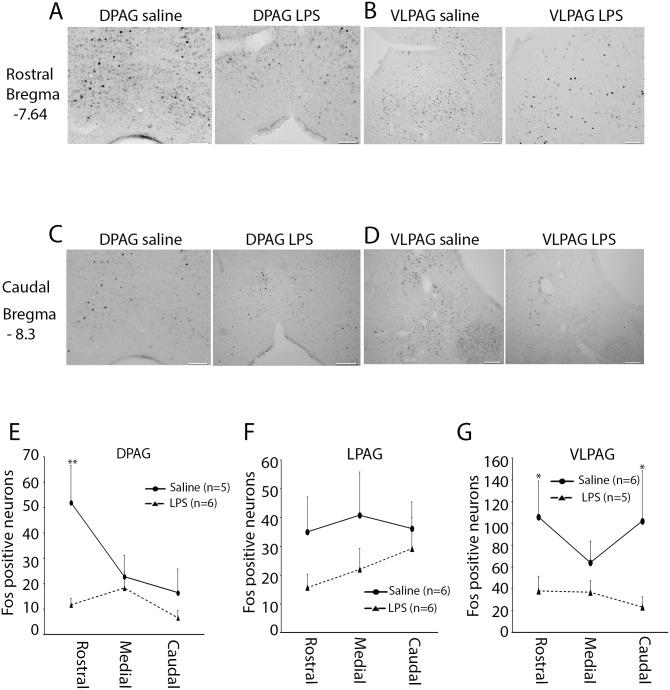
Fos-protein expression in the PAG was examined in the three main subdivisions (DPAG; LPAG; and VLPAG) and across three rostrocaudal axes (rostral, medial, and caudal). In the DPAG, LMM analysis revealed a significant effect of treatment [F(1,25) = 7.52; p<.05] within the DPAG at the rostral level. Pairwise comparisons revealed that neonatal saline-treated rats displayed significantly higher numbers of Fos-positive cells compared to neonatal LPS-treated rats following formalin injection (p<.01). No significant differences were found in the medial and caudal part of the PAG (Figure 2E, Table 1).
Figure 2. Fos immunoreactivity in the midbrain periaqueductal grey (PAG) following a neonatal immune challenge and subsequent inflammatory pain.
Representative examples illustrate the distribution of Fos-positive nuclei in the dorsal and ventrolateral PAG (DPAG, VLPAG, respectively) at the rostral (A & B) and caudal (C & D) axes following formalin injection in preadolescent rats. (E) Quantification of Fos-positive nuclei in DPAG between neonatal saline and LPS-treated rats. (F) Quantification of Fos-positive nuclei in lateral PAG (LPAG). (G) Quantification of Fos-positive nuclei in VLPAG. Data are presented as mean ± SEM. *p<.05; **p<.01. Scale bar = 100 µm.
Table 1. Fos expression in the periaqueductal grey (PAG).
| Treatment | Axis | DPAG | LPAG | VLPAG |
| Sal/formalin | Rostral | 52.0±14.5 | 35.0±12.2 | 106.0±33.1 |
| Medial | 22.8±8.3 | 40.8±14.9 | 64.0±19.7 | |
| Caudal | 16.5±10.2 | 36.2±9.1 | 102.2±46.3 | |
| LPS/formalin | Rostral | 11.6±2.4 | 15.6±4.6 | 38.0±13.6 |
| Medial | 18.3±6.0 | 22.0±7.2 | 36.8±10.6 | |
| Caudal | 6.6±3.1 | 29.2±4.9 | 23.2±9.3 |
Note: Data are presented as mean number of Fos-positive cells per section ± SEM.
No significant differences were found in terms of Fos-positive nuclei on the ipsilateral versus contralateral side of either the LPAG or the VLPAG, and therefore these data were combined. In the LPAG, LMM analysis revealed a strong trend towards decreased Fos-positive neurons in LPS-treated rats, however this did not reach significance (p = .062; Figure 2F). In contrast, there was a significant main effect of treatment on the induction of Fos-protein in both the rostral and caudal VLPAG [F(1,34) = 5.71; p<.05; F(1,34) = 8.39; p<.01, respectively]. Pairwise comparisons revealed that neonatal saline-treated rats exhibited significantly higher Fos-positive neurons compared to LPS animals in the rostral (p = .05) and caudal (p<.05) VLPAG (Figure 2G, Table 1).
Bilateral Fos-protein labelling was observed in the LHb and medial Habenula (MHb). No significant differences were observed in Fos-like immunoreactivity in the ipsilateral versus contralateral sides of formalin injection, and therefore data from both sides were combined. LMM analysis revealed a significant main effect of subregion [F(1,18) = 46.17; p<.001] implying that the LHb and MHb showed different pattern of Fos activation. Pairwise comparisons indicated that regardless of the neonatal challenge, significantly greater levels of Fos-protein expression were observed in the LHb compared to the MHb following formalin injection (p<.001; Figure 3A & 3B).
Figure 3. Fos immunoreactivity in the habenula following a neonatal immune challenge and subsequent inflammatory pain.
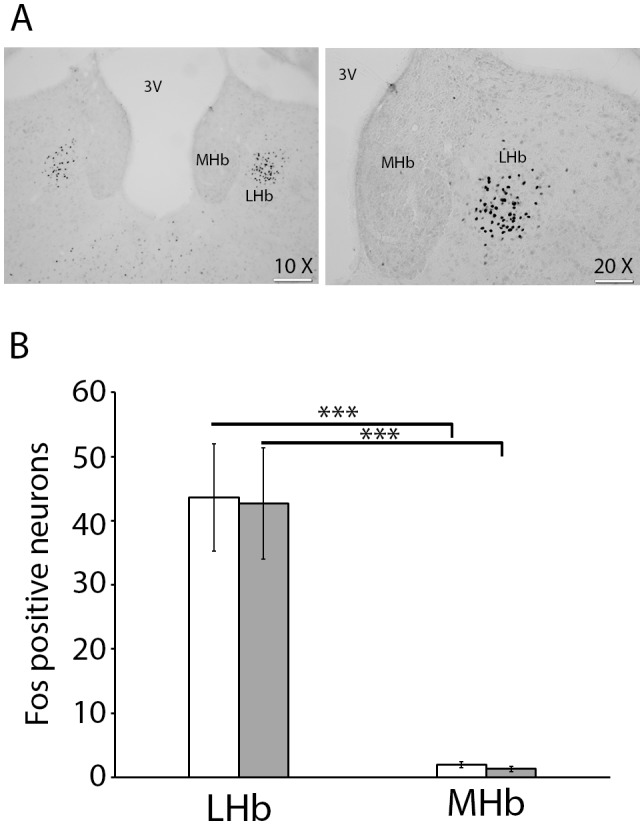
(A) Representative examples illustrate the distribution of Fos-positive nuclei in the lateral and medial habenula (LHb & MHb, respectively). (B) Quantification of Fos-positive nuclei in LHb and MHb in neonatal saline (white bar) and LPS-treated rats (grey bar) after formalin injection in preadolescent rats. 3V: third ventricle. ***p<.001. Scale bar = 100 µm for the 10x microscopic objective and 200 µm for 20x microscopic objective.
The PVT is known to express Fos following formalin injection into the hindpaw in rats [22]. There was a trend towards increased levels of Fos immunoreactivity in the PVT in neonatal saline-treated animals compared to LPS-treated animals, however this failed to reach significance (p = .175) (Table 2).
Table 2. Fos expression in the paraventricular nucleus of the thalamus (PVT) and the lateral and medial habenula (LHb & MHb).
| Treatment | PVT | LHb | MHb |
| Sal/formalin | 22.1±6.0 | 43.6±8.3 | 1.9±0.4 |
| LPS/formalin | 14.3±2.1 | 42.7±8.6 | 1.2±0.3 |
Note: Data are presented as mean number of Fos-positive cells per section ± SEM.
Discussion
The current study demonstrates that exposure to an immune challenge during the neonatal period is accompanied by enhanced behavioural responses to formalin in preadolescent rats. We observed increased susceptibility of preadolescent rats to formalin-induced flinching but not licking behaviours in LPS-treated rats. This LPS-induced hyperalgesia was associated with distinct recruitment of supra-spinal structures as indicated by significantly attenuated Fos-protein expression in the rostral DPAG as well as rostral and caudal axes of the VLPAG.
Neonatal Exposure to LPS Alters Flinching but not Licking Responses in Preadolescent Rats
The nociceptive system undergoes fine-tuning and maturation during the first postnatal weeks of development [54]. While C-fibers are present within the spinal cord prenatally, they become functional and respond to noxious stimuli only during the second postnatal week. For instance, the ability of C-fibers to produce neurogenic oedema appears only at PND 11–14 [55]. Moreover, application of mustard oil, a specific C-fibers irritant, to the hindlimb skin does not induce Fos-protein expression in the spinal cord until the second week of development [56]. These findings suggest that the nociceptive circuitry is highly malleable during early postnatal life. Therefore, exposure to a physiological insult such as LPS during the first weeks of postnatal life, where the neurocircuitry underlying nociception undergoes significant plasticity, is likely to interfere with the normal developmental trajectory of the nociceptive system, leading to altered behavioural and neuronal responses following exposure to noxious stimuli later in development.
The current study demonstrated that neonatal LPS exposure produced increased flinching but not licking during both phases of the formalin test. These findings are in accordance with our previous work whereby neonatal LPS exposure was able to alter flinching but not licking patterns in preadolescent rats [10]. Flinching and licking are mediated by distinct neural pathways. Whilst the spontaneous rhythmicity of flinching behaviour suggests this behaviour is spinally modulated, the complex movements involved in licking behaviour suggest it engages supraspinal modulation. Indeed, intrathecal injection of Lidocaine into the lumbar spinal cord prior to formalin injection abolishes flinching response during the late phase [57]. However, transection of the spinal cord at the mid-thoracic level has been shown to have no effect on formalin-induced flinching [57], but completely abolishes formalin-induced licking [43]. The findings reported in these animal studies suggest that the generation of flinching and licking behaviours result from the activation of distinct neural pathways. Exposure to an immune challenge is thus able to alter either the spinal or supraspinal circuitry leading to alteration in the intensity and profile of the biphasic formalin response later in development. Together, the current findings suggest that neonatal exposure to LPS enhances the sensitivity of preadolescent rats to inflammatory noxious stimuli (i.e. hyperalgesia). This is in accordance with a recent study demonstrating that rats challenged with LPS at PND 14 displayed enhanced mechanical and thermal nociception when tested in adulthood (8 to 12 weeks old) [58]. In this study a subsequent exposure to LPS in adulthood failed to alter the behavioural responses to mechanical and thermal stimuli [58]. These data suggest that a single immune challenge is sufficient to disrupt nociception later in life. The current findings add to the literature by demonstrating that a neonatal immune challenge can also alter inflammatory nociception as indicated by enhanced formalin-induced flinching in preadolescent rats.
Neonatal LPS Exposure Results in Distinct Recruitment of Supra-spinal Structures Involved in Analgesia following Formalin Injection
Electrical or chemical stimulation of the PAG produces profound analgesia in rats [18], [20], [25], [36], [59], [60]. The PAG is organized into longitudinal columns [24], [61], with a dorsal, lateral, and ventrolateral column. The current study demonstrates that in response to a neonatal immune challenge and subsequent inflammatory pain at PND 22, preadolescent rats exhibited distinct patterns of Fos-protein expression within these columns. At PND 22, following formalin injection, LPS-challenged rats exhibited significantly attenuated levels of Fos-protein in both rostral DPAG and rostral and caudal VLPAG compared to saline-challenged rats. Interestingly, although there was a strong trend towards reduced Fos-protein expression in the LPAG in LPS-treated rats, this did not reach statistical significance, possibly due to the lack of power in our analyses. These findings are in agreement with recent observations showing significantly increased Fos immunolabelling in the caudal part of the DPAG and VLPAG following formalin injection in PND 14 rats [22]. Electrical or opiate stimulation of the DPAG and VLPAG has been previously reported to induce analgesia in infant and adult rats [62]–[64]. For instance, injection of the µ-opiate agonist, DAMGO into both ventral and dorsal PAG was associated with enhanced paw withdrawal latency to a noxious thermal stimulus (47°C water) in 3, 10, and 14 day-old rats [63]. Since stimulation of both the VLPAG and DPAG mediates analgesia [25], [65], the reduction in Fos-labelling within the DPAG and VLPAG of LPS-challenged rats is likely associated with the observed hyperalgesia in these animals following the formalin injection.
In addition to the PAG, the LHb has also been demonstrated to play an important role in mediating analgesia in the formalin test [32], [33]. Further, increased neuronal depolarization was observed in the LHb in response to noxious peripheral stimulation [30]. Importantly, these effects appear to be specific to the LHb, as adult rats injected with formalin have been previously shown to display increased Fos labelling in the LHb but not the MHb [34]. Moreover, electrical stimulation of the LHb but not the MHb in freely moving rats has been shown to evoke naloxone-reversible analgesia in the tail flick test [66]. Together, these findings suggest that compared to the MHb, neurons in the LHb respond specifically to noxious stimulations. This is consistent with our findings where formalin injection into the hindpaw of preadolescent rats was associated with increased Fos-protein labelling in the LHb as compared to MHb. However, in the current study, the intensity of Fos-protein expression was not affected by neonatal immune challenge. It is however possible that formalin injection resulted in maximal Fos-protein expression in LHb across both groups, thereby preventing the identification of a treatment effect.
Morphological studies have revealed the existence of afferent and efferent connections between the habenula and the PAG [28], [34], [67], [68]. The habenula constitutes an important relay in the descending pathway to the PAG to subserve antinociception [69], [70]. Administration of morphine into the habenula of a rabbit produced a marked increase in the withdrawal reflex latency to radiant heat [69]. This antinociception was dose-dependently attenuated by intra-PAG administration of the opiate antagonist naloxone [69]. Furthermore, the antinociception induced by intra-habenula injection of morphine, was attenuated by muscimol (a GABA receptor agonist) injected into the PAG [69]. These data suggest that morphine can act on the habenula to activate a neural descending pathway projecting to the PAG to mediate analgesia. This pathway seems to implicate the release of endogenous opioid peptides and GABA. Of particular interest, stimulation of the LHb was reported to inhibit the unit discharges of nociceptive-specific neurons within the PAG [71]. The findings reported in these animal studies are consistent with a recent human study that revealed, using fMRI technique, an interrelated activity of habenula and PAG following noxious thermal stimulation [70].
Since no significant differences were observed in the LHb, it is possible that an alternate structure innervated the PAG to mediate the decreased behavioural responses to formalin observed in control animals. Another possibility is that the analgesia observed in control animals could be attributed to PAG activation alone. Further research is required to examine these possibilities.
Conclusion
The ability to perceive and respond to noxious stimuli is critical for survival. Equally important is the recruitment of supra-spinal structures involved in analgesia to reduce the suffering associated with pain. The current study importantly demonstrates that neonatal exposure to LPS results in decreased neuronal activation within the PAG following exposure to a noxious inflammatory stimulus in preadolescence. We have also demonstrated that neonatal exposure to LPS produces altered behavioural responses to formalin injection in preadolescent rats. These findings highlight the importance of neonatal immune challenge in programming behavioural and supra-spinal responses to inflammatory pain later in development.
Acknowledgments
We would like to thank all conjoint BSAF staff for their assistance in animal husbandry. We also acknowledge Kim Colyvas for his assistance with statistical analysis.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data will be available on Plos One website upon acceptance.
Funding Statement
This study was supported by a grant from the Australian Research Council (ARC DP 09787599). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Beggs S, Currie G, Salter MW, Fitzgerald M, Walker SM (2012) Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain : a journal of neurology 135: 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T (2000) Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science 289: 628–631. [DOI] [PubMed] [Google Scholar]
- 3. Walker SM, Tochiki KK, Fitzgerald M (2009) Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain 147: 99–106. [DOI] [PubMed] [Google Scholar]
- 4. Laprairie JL, Johns ME, Murphy AZ (2008) Preemptive morphine analgesia attenuates the long-term consequences of neonatal inflammation in male and female rats. Pediatric research 64: 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, et al. (2004) Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain 110: 588–596. [DOI] [PubMed] [Google Scholar]
- 6. Goffaux P, Lafrenaye S, Morin M, Patural H, Demers G, et al. (2008) Preterm births: can neonatal pain alter the development of endogenous gating systems? Eur J Pain 12: 945–951. [DOI] [PubMed] [Google Scholar]
- 7. Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, et al. (2000) Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proceedings of the National Academy of Sciences of the United States of America 97: 5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker AK, Hawkins G, Sominsky L, Hodgson DM (2012) Transgenerational transmission of anxiety induced by neonatal exposure to lipopolysaccharide: implications for male and female germ lines. Psychoneuroendocrinology 37: 1320–1335. [DOI] [PubMed] [Google Scholar]
- 9. Walker FR, Hodyl NA, Hodgson DM (2009) Neonatal bacterial endotoxin challenge interacts with stress in the adult male rat to modify KLH specific antibody production but not KLH stimulated ex vivo cytokine release. Journal of neuroimmunology 207: 57–65. [DOI] [PubMed] [Google Scholar]
- 10. Zouikr I, Tadros MA, Barouei J, Beagley KW, Clifton VL, et al. (2014) Altered nociceptive, endocrine, and dorsal horn neuron responses in rats following a neonatal immune challenge. Psychoneuroendocrinology 41: 1–12. [DOI] [PubMed] [Google Scholar]
- 11. Hodgson DM, Knott B, Walker FR (2001) Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatric research 50: 750–755. [DOI] [PubMed] [Google Scholar]
- 12. Shanks N, Larocque S, Meaney MJ (1995) Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 15: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walker FR, Hodyl NA, Krivanek KM, Hodgson DM (2006) Early life host-bacteria relations and development: long-term individual differences in neuroimmune function following neonatal endotoxin challenge. Physiology & behavior 87: 126–134. [DOI] [PubMed] [Google Scholar]
- 14. Gebhart GF (2004) Descending modulation of pain. Neuroscience and biobehavioral reviews 27: 729–737. [DOI] [PubMed] [Google Scholar]
- 15. Ren K, Dubner R (2002) Descending modulation in persistent pain: an update. Pain 100: 1–6. [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald M, Koltzenburg M (1986) The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain research 389: 261–270. [DOI] [PubMed] [Google Scholar]
- 17. Fardin V, Oliveras JL, Besson JM (1984) A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain research 306: 105–123. [DOI] [PubMed] [Google Scholar]
- 18. Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC (1971) Analgesia from electrical stimulation in the brainstem of the rat. Science 174: 1351–1354. [DOI] [PubMed] [Google Scholar]
- 19. Oliveras JL, Besson JM, Guilbaud G, Liebeskind JC (1974) Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Experimental brain research 20: 32–44. [DOI] [PubMed] [Google Scholar]
- 20. Reynolds DV (1969) Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164: 444–445. [DOI] [PubMed] [Google Scholar]
- 21. Baulmann J, Spitznagel H, Herdegen T, Unger T, Culman J (2000) Tachykinin receptor inhibition and c-Fos expression in the rat brain following formalin-induced pain. Neuroscience 95: 813–820. [DOI] [PubMed] [Google Scholar]
- 22. Barr GA (2011) Formalin-induced c-fos expression in the brain of infant rats. The journal of pain : official journal of the American Pain Society 12: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaccarino AL, Chorney DA (1994) Descending modulation of central neural plasticity in the formalin pain test. Brain research 666: 104–108. [DOI] [PubMed] [Google Scholar]
- 24. Bandler R, Shipley MT (1994) Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends in neurosciences 17: 379–389. [DOI] [PubMed] [Google Scholar]
- 25. Cannon JT, Prieto GJ, Lee A, Liebeskind JC (1982) Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain research 243: 315–321. [DOI] [PubMed] [Google Scholar]
- 26. Carstens E, Douglass DK (1995) Midbrain suppression of limb withdrawal and tail flick reflexes in the rat: correlates with descending inhibition of sacral spinal neurons. Journal of neurophysiology 73: 2179–2194. [DOI] [PubMed] [Google Scholar]
- 27. Nichols DS, Thorn BE (1990) Stimulation-produced analgesia and its cross-tolerance between dorsal and ventral PAG loci. Pain 41: 347–352. [DOI] [PubMed] [Google Scholar]
- 28. Li YQ, Takada M, Mizuno N (1993) Demonstration of habenular neurons which receive afferent fibers from the nucleus accumbens and send their axons to the midbrain periaqueductal gray. Neuroscience letters 158: 55–58. [DOI] [PubMed] [Google Scholar]
- 29. Ito M, Kadekaro M, Sokoloff L (1985) Effects of lateral habenular lesions on local cerebral glucose utilization in the rat. Brain research 337: 245–254. [DOI] [PubMed] [Google Scholar]
- 30. Dafny N, Qiao JT (1990) Habenular neuron responses to noxious input are modified by dorsal raphe stimulation. Neurological research 12: 117–121. [DOI] [PubMed] [Google Scholar]
- 31. Herkenham M, Nauta WJ (1977) Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. The Journal of comparative neurology 173: 123–146. [DOI] [PubMed] [Google Scholar]
- 32. Cohen SR, Melzack R (1985) Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain research 359: 131–139. [DOI] [PubMed] [Google Scholar]
- 33. Cohen SR, Melzack R (1986) Habenular stimulation produces analgesia in the formalin test. Neuroscience letters 70: 165–169. [DOI] [PubMed] [Google Scholar]
- 34. Nagao M, Kamo H, Akiguchi I, Kimura J (1993) Induction of c-Fos-like protein in the lateral habenular nucleus by persistent noxious peripheral stimulation. Neuroscience letters 151: 37–40. [DOI] [PubMed] [Google Scholar]
- 35. Basbaum AI, Fields HL (1978) Endogenous pain control mechanisms: review and hypothesis. Annals of neurology 4: 451–462. [DOI] [PubMed] [Google Scholar]
- 36. Basbaum AI, Fields HL (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annual review of neuroscience 7: 309–338. [DOI] [PubMed] [Google Scholar]
- 37. Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, et al. (2009) Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology 34: 1515–1525. [DOI] [PubMed] [Google Scholar]
- 38. Walker FR, Knott B, Hodgson DM (2008) Neonatal endotoxin exposure modifies the acoustic startle response and circulating levels of corticosterone in the adult rat but only following acute stress. Journal of psychiatric research 42: 1094–1103. [DOI] [PubMed] [Google Scholar]
- 39. Nilsson C, Jennische E, Ho HP, Eriksson E, Bjorntorp P, et al. (2002) Postnatal endotoxin exposure results in increased insulin sensitivity and altered activity of neuroendocrine axes in adult female rats. European journal of endocrinology/European Federation of Endocrine Societies 146: 251–260. [DOI] [PubMed] [Google Scholar]
- 40. Dubuisson D, Dennis SG (1977) The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4: 161–174. [DOI] [PubMed] [Google Scholar]
- 41. Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992) The formalin test: an evaluation of the method. Pain 51: 5–17. [DOI] [PubMed] [Google Scholar]
- 42. Zouikr I, Tadros MA, Clifton VL, Beagley KW, Hodgson DM (2013) Low formalin concentrations induce fine-tuned responses that are sex and age-dependent: a developmental study. PloS one 8: e53384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wheeler-Aceto H, Cowan A (1991) Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology 104: 35–44. [DOI] [PubMed] [Google Scholar]
- 44. Nadel L (1990) Varieties of spatial cognition. Psychobiological considerations. Annals of the New York Academy of Sciences 608: 613–626 discussion 626–636 [DOI] [PubMed] [Google Scholar]
- 45. Butkevich IP, Vershinina EA (2001) Prenatal stress alters time characteristics and intensity of formalin-induced pain responses in juvenile rats. Brain research 915: 88–93. [DOI] [PubMed] [Google Scholar]
- 46. Guy ER, Abbott FV (1992) The behavioral response to formalin in preweanling rats. Pain 51: 81–90. [DOI] [PubMed] [Google Scholar]
- 47. Okuda K, Sakurada C, Takahashi M, Yamada T, Sakurada T (2001) Characterization of nociceptive responses and spinal releases of nitric oxide metabolites and glutamate evoked by different concentrations of formalin in rats. Pain 92: 107–115. [DOI] [PubMed] [Google Scholar]
- 48. Yi DK, Barr GA (1995) The induction of Fos-like immunoreactivity by noxious thermal, mechanical and chemical stimuli in the lumbar spinal cord of infant rats. Pain 60: 257–265. [DOI] [PubMed] [Google Scholar]
- 49. Kovacs KJ (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochemistry international 33: 287–297. [DOI] [PubMed] [Google Scholar]
- 50. Wiedenmayer CP, Barr GA (2001) Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behavioural brain research 126: 147–157. [DOI] [PubMed] [Google Scholar]
- 51. Loyd DR, Murphy AZ (2009) The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? Neural plasticity 2009: 462879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F (2008) Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biological psychiatry 63: 152–157. [DOI] [PubMed] [Google Scholar]
- 53. James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience 199: 235–242. [DOI] [PubMed] [Google Scholar]
- 54. Fitzgerald M (2005) The development of nociceptive circuits. Nature reviews Neuroscience 6: 507–520. [DOI] [PubMed] [Google Scholar]
- 55. Fitzgerald M, Gibson S (1984) The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience 13: 933–944. [DOI] [PubMed] [Google Scholar]
- 56. Williams S, Evan G, Hunt SP (1990) Spinal c-fos induction by sensory stimulation in neonatal rats. Neuroscience letters 109: 309–314. [DOI] [PubMed] [Google Scholar]
- 57. Coderre TJ, Yashpal K, Henry JL (1994) Specific contribution of lumbar spinal mechanisms to persistent nociceptive responses in the formalin test. Neuroreport 5: 1337–1340. [PubMed] [Google Scholar]
- 58. Boisse L, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ (2005) Neonatal immune challenge alters nociception in the adult rat. Pain 119: 133–141. [DOI] [PubMed] [Google Scholar]
- 59. Jacquet YF, Lajtha A (1974) Paradoxical effects after microinjection of morphine in the periaqueductal gray matter in the rat. Science 185: 1055–1057. [DOI] [PubMed] [Google Scholar]
- 60. Morgan MM, Sohn JH, Liebeskind JC (1989) Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain research 502: 61–66. [DOI] [PubMed] [Google Scholar]
- 61. Beitz AJ (1985) The midbrain periaqueductal gray in the rat. I. Nuclear volume, cell number, density, orientation, and regional subdivisions. The Journal of comparative neurology 237: 445–459. [DOI] [PubMed] [Google Scholar]
- 62. Barr GA, Miya DY, Paredes W (1992) Analgesic effects of intraventricular and intrathecal injection of morphine and ketocyclazocine in the infant rat. Brain research 584: 83–91. [DOI] [PubMed] [Google Scholar]
- 63. Barr GA, Wang S (2013) Analgesia induced by localized injection of opiate peptides into the brain of infant rats. Eur J Pain 17: 676–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. D'Mello R, Dickenson AH (2008) Spinal cord mechanisms of pain. British journal of anaesthesia 101: 8–16. [DOI] [PubMed] [Google Scholar]
- 65. Morgan MM, Gold MS, Liebeskind JC, Stein C (1991) Periaqueductal gray stimulation produces a spinally mediated, opioid antinociception for the inflamed hindpaw of the rat. Brain research 545: 17–23. [DOI] [PubMed] [Google Scholar]
- 66. Mahieux G, Benabid AL (1987) Naloxone-reversible analgesia induced by electrical stimulation of the habenula in the rat. Brain research 406: 118–129. [DOI] [PubMed] [Google Scholar]
- 67. Aghajanian GK, Wang RY (1977) Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain research 122: 229–242. [DOI] [PubMed] [Google Scholar]
- 68. Araki M, McGeer PL, Kimura H (1988) The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain research 441: 319–330. [DOI] [PubMed] [Google Scholar]
- 69. Yu LC, Han JS (1990) Habenula as a relay in the descending pathway from nucleus accumbens to periaqueductal grey subserving antinociception. The International journal of neuroscience 54: 245–251. [DOI] [PubMed] [Google Scholar]
- 70. Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, et al. (2012) Mapping pain activation and connectivity of the human habenula. Journal of neurophysiology 107: 2633–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang S, Fang JZ, Xia YH (1984) The effect of stimulating nucleus accumbens on the discharge of pain-related neurons in the periaqueductal grey. Physiological Science 4: 37–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data will be available on Plos One website upon acceptance.