Abstract
Despite the high prevalence of obstructive sleep apnea (OSA) in type 2 diabetes mellitus (DM), the attributable vascular risk from each condition is unknown. We hypothesize that OSA may have a similar effect on vascular function as type 2 diabetes does. Healthy normal-weight subjects, healthy obese subjects, subjects with type 2 diabetes, and obese subjects with OSA were enrolled. Vascular function was assessed with brachial artery ultrasound for flow-mediated dilatation (FMD) and in skin microcirculation by laser Doppler flowmetry. One hundred fifty-three subjects were studied: healthy normal-weight controls (NCs) (n = 14), healthy obese controls (OCs) (n = 33), subjects with DM (n = 68), and obese subjects with OSA (n = 38). The DM group did not undergo sleep study and thus may have had subclinical OSA. The OSA and type 2 diabetes groups had impaired FMD as compared to both the normal-weight and OC groups (5.8 ± 3.8%, 5.4 ± 1.6% vs. 9.1 ± 2.5%, 8.3 ± 5.1%, respectively, P < 0.001, post hoc Fischer test). When referenced to the NC group, a multiple linear regression model adjusting for covariates found that baseline brachial artery diameter (β = −3.75, P < 0.001), OSA (β = −2.45, P = 0.02) and type 2 diabetes status (β = −2.31, P = 0.02), negatively predicted % FMD. OSA status did not seem to affect nitroglycerin-induced vasodilation (endothelium-independent) of the brachial artery or vascular function in the skin microcirculation. OSA impairs endothelial function in the brachial artery to a similar degree as type 2 diabetes does. OSA, however, does not appear to affect brachial endothelium-independent vasodilation or skin microcirculatory function. Treatment of OSA in patients with concomitant type 2 diabetes, therefore, may be a potential therapeutic option to improve macro-, but not microvascular outcomes.
INTRODUCTION
The recent obesity epidemic has been blamed for the increasing prevalence of type 2 diabetes mellitus (DM) and obstructive sleep apnea (OSA) (1–5). Type 2 DM is an important cause of cardiovascular disease resulting in major morbidity and mortality. Although intensive glycemic control in the treatment of type 2 diabetes has been shown to improve microvascular complications, refinements in glycemic control have not been shown to prevent mortality from macrovascular complications (6). Therefore, alternative therapeutic pathways need to be explored to lower the macrovascular complications of DM further (7).
Although once thought to be independent diseases, the high prevalence of OSA among patients with type 2 diabetes (8,9) and vice versa (10) has raised interesting questions as to how OSA and DM interact. OSA is a disorder characterized by upper airway collapse causing sleep fragmentation, repetitive hypoxemia, and sympathetic surges (11,12). Recently, OSA has been associated with cardiovascular sequelae such as hypertension (13), stroke (14), ischemic heart disease, and cardiac arrhythmias (15). Because obesity is a risk factor for both OSA and type 2 diabetes, coexistence of OSA and DM was initially attributed to common comorbidities. However, patients with OSA, independent of obesity, have been found to have impaired glucose tolerance (16). Furthermore, sleep deprivation and disturbance, which are characteristics of OSA, have been linked to risk of incident diabetes. Patients with DM, on the other hand, have been found to be at increased risk of sleep-disordered breathing, perhaps as a result of diabetic autonomic neuropathy (17). In a recent study, ~90% of subjects with type 2 DM were diagnosed with OSA (9). Furthermore, preliminary results indicate that a year after OSA was diagnosed, only 2% of subjects were evaluated for treatment. Although the causative mechanisms linking OSA and DM still need to be clarified, there is clearly a large overlap between the two epidemics.
OSA, like DM (18), has been associated with impairments in endothelial function in patients without overt cardiovascular disease (19). Endothelial dysfunction is likely an early manifestation of atherosclerosis (20). Because OSA and DM often occur concomitantly, treatment of OSA may improve vascular outcomes in patients with both OSA and DM. In addition, although several studies have examined endothelial-dependent and -independent macrovascular function in OSA, minimal research has examined the microcirculation in OSA, leaving the most appropriate design of a therapeutic study of OSA in DM unclear.
In order to understand the roles of OSA and DM on macrovascular and microcirculatory function, we compared populations of patients with OSA to patients with DM in a physiological study. We sought to test the hypothesis that the effect of OSA on vascular function would be similar to DM and that the observed effect would be independent of obesity. Prior to embarking on a therapeutic trial for OSA in DM, we compared the magnitude and nature of the vascular effect of OSA to that of DM to allow us to design a clinical trial more rigorously.
METHODS AND PROCEDURES
Study subjects
Individuals were recruited through Institutional Review Board–approved protocols after providing informed consent. Subjects at high risk for OSA, with a BMI ≥30 kg/m2 but without other comorbid conditions, were recruited from the community through advertisements. Subjects with type 2 diabetes were recruited from endocrine and podiatry clinics. They were identified as having type 2 diabetes, according to American Diabetes Association criteria. Healthy normal-weight control (NC) subjects were recruited from the community through advertisements. Although some of our subjects had participated in prior studies, none of the results of the present study is previously reported.
Selection and exclusion criteria
Subjects 18–80 years old were included in the study and were divided into four groups: healthy NCs (n = 14), healthy obese controls (OCs, n = 33), subjects with DM (n = 68), and obese subjects with OSA (OSA, n = 38). Healthy subjects, OCs, and obese OSA patients underwent a fasting glucose test to assess for occult diabetes. OC subjects and obese subjects with OSA had attended overnight polysomnograms, whereas normal healthy lean controls and DM patients did not have polysomnography. Although people with DM did not undergo polysomnography, they did not have clinical sleep apnea diagnosed or treated, but may have had subclinical or occult sleep apnea. Obese subjects with and without OSA, with a BMI ≥30 kg/m2 were recruited mainly from the community through paper and Internet advertisements. OSA was defined as an apnea-hypopnea index ≥10/h of sleep. Exclusion criteria for participants were as follows: cigarette smoking, congestive heart failure, cardiac arrhythmias, stroke or transient ischemic attack, end stage renal failure, uncontrolled hypertension, severe dyslipidemia, or any other severe chronic medical condition requiring active treatment. Furthermore, in order to avoid any influence on brachial artery diameter by antihypertensive or lipid-lowering medications, subjects had to be on stable treatment over the prior 6 months. Among the OCs, two were on antihypertensive medication and one took a statin. Among the OSA participants, two were on anti-hypertensive, one statin, and one metformin (nondiabetic). Among the type 2 DM patients, the majority were on oral agents; however, of these 68 participants, 7 were on no medications and 23 were on insulin (with or without oral agents).
Clinical measurements
All clinical examinations and evaluations were conducted during the morning hours after an overnight fast. Subjects were abstinent from alcohol or vigorous exercise 24 h prior to testing. Vital signs were measured by experienced physicians and nurses. Each subject underwent a thorough medical history and physical examination by a licensed physician.
Blood samples were obtained using standard sterile technique from the antecubital vein. Plasma glucose, total serum cholesterol, and triglycerides were measured using the Synchoron CX analyzer (Beckman Systems, Fullerton, CA). High-density lipoprotein serum cholesterol was measured directly (Sigma, St Louis, MO) and low-density lipoprotein was calculated. The glycated hemoglobin was measured in whole blood with ion-exchange high-performance liquid chromatography. Complete blood counts were measured at Beth Israel Deaconess Medical Center’s core laboratory using standard laboratory techniques.
Vascular reactivity measurements
All vascular reactivity measurements were made while the subjects were fasting during the morning hours of testing. All studies were performed in temperature-controlled rooms (24–26 °C). The vascular reactivity of the skin microcirculation was measured using laser Doppler flowmetry before and after the iontophoresis of acetylcholine (Ach, endothelium-dependent vasodilation) and sodium nitroprusside (SNp, endothelium-independent vasodilation). All measurements were taken from the ventral surface of the forearm. The reproducibility of this technique has been previously described (21).
Vascular reactivity of the macrocirculation was measured in the brachial artery using a high-resolution ultrasound with a 10.0 MHz linear array transducer and an HDI Ultramark 9 system (Advanced Technology Laboratories, Bothell, Washington). To measure endothelial-dependent vasodilation, the brachial artery diameter was measured before and after flow-mediated dilation (FMD) during reactive hyperemia. Reactive hyperemia was accomplished by inflating a pneumatic tourniquet distal to the brachial artery to 50 mm Hg above systolic blood pressure for 5 min and then deflating it. This method has been described previously and performed according to published guidelines (18). To measure endothelial-independent vasodilation, the brachial artery width was measured before and after administration of systemic sublingual nitroglycerin (400 mcg). Ultrasound images were recorded on super-VHS videotape and a reader blinded to study results interpreted the images. The coefficient of variation of the vascular reactivity measurements in our laboratory, which is <15%, has been described (18).
Polysomnogram
Recorded signals included the following: electroencephalogram (C4-A1, C3-A2, O2-A1, and O1-A2), left and right electro-oculogram, submental and bilateral tibial electromyogram, surface electrocardiogram, airflow, chest and abdominal excursion (piezo bands), oxyhemoglobin saturation, and body position. All data were collected on Nihon Kohden digital polysomnography (Nihon Kohden, Tokyo, Japan). Polysomnograms were scored by a blinded experienced registered sleep technologist and scored according to standard American Academy of Sleep Medicine criteria (22). An apnea was scored if airflow was absent for 10 s, and a hypopnea was scored if there was at least a 50% reduction in airflow for 10 s or a detectable decrement in airflow for 10 s in association with either an oxyhemoglobin desaturation of at least 3% or an arousal. An apnea-hypopnea index was then calculated based on the number of apneas and hypopneas per hour of sleep.
Statistical analysis
The Minitab 15 statistical package (Minitab, State College, PA) for personal computers was used for the statistical analysis. For normally distributed data, ANOVA was used followed by the post hoc Fisher’s test to identify differences between groups. For categorical data, Pearson’s χ2 tests were used. For nonparametrically distributed data, the Kruskal–Wallis test was used. Although univariate analyses were performed, the nature of our participants is that multiple potential covariates exist. To adjust for these potential covariates (age, BMI, sex, and baseline vascular measurements) and avoid overfitting, a step-forward multiple linear regression model with a stay criterion of 0.10 was built to determine predictors of outcome measures (%FMD, %NID, %Ach, and %SNp). Dummy variables were created for OC, type 2 DM, and the OSA groups with the NC group as the reference category (to account for groups). Predictors that were significant in the step-forward multiple linear regression model were then included in a linear regression model with dummy variables (OC, type 2 diabetes, and OSA) forced into the model (to account for groups). Two-sided P values <0.05 were considered statistically significant.
RESULTS
Characteristics of study participants
The baseline characteristics of the 153 subjects studied are listed in Table 1. Compared to OC and OSA subjects, the DM group was older, had higher blood pressure, glycated hemoglobin, and fasting glucose but lower low-density lipoprotein levels (Table 1). The NC group was similar in age to the DM group but older than both the OSA and OC groups. By study design, the OSA and OC groups had a higher BMI and increased waist-to-hip ratios compared to the NC and DM groups (Table 1).
Table 1.
Baseline characteristics of study participants
| Normal-weight control (NC) n = 14 |
Obese control (OC) n = 33 |
Diabetes mellitus (type 2 diabetes) n = 68 |
Obstructive sleep apnea (OSA) n = 38 |
|
|---|---|---|---|---|
| Male (%) | 3 (21%) | 9 (27%) | 37 (54%) | 20 (53%) |
| Age (years) | 59 ± 7 | 33 ± 12 | 58 ± 9 | 44 ± 10 |
| BMI (kg/m2) | 23 (20, 27) | 38 (34, 43) | 34 (31, 39) | 37 (33, 45) |
| Waist:hip ratio | 0.83 ± 0.06 | 0.86 ± 0.10 | 0.95 ± 0.08 | 0.94 ± 0.09 |
| SBP (mm Hg) | 129 ± 23 | 123 ± 12 | 140 ± 21 | 132 ± 13 |
| DBP (mm Hg) | 73 ± 14 | 70 ± 8 | 76 ± 11 | 79 ± 10 |
| Total cholesterol (mg/dl) | 208 (175, 219) | 179 (156, 213) | 170 (150, 193) | 193 (173, 220) |
| HDL (mg/dl) | 73 (51, 84) | 51 (44, 69) | 49 (41, 55) | 50 (41, 58) |
| LDL (mg/dl) | 112 (93, 123) | 99 (91, 129) | 87 (66, 173) | 113 (93, 187) |
| HbA1c (%) | 5.6 (5.5, 5.8) | 5.5 (5.1, 5.6) | 7.2 (6.4, 8.6) | 5.6 (5.4, 6.0) |
| Fasting glucose (mg/dl) | 78 (71, 87) | 79 (71, 88) | 125 (90, 166) | 82 (77, 95) |
For normally distributed data, means ± standard deviations are displayed. For nonparametrically distributed data, medians (interquartile ranges) are displayed.
DBP, diastolic blood pressure; HbA1c, glycated hemoglobin, type A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OSA, obstructive sleep apnea; SBP, systolic blood pressure.
A total of 71 obese subjects without diabetes were evaluated for OSA. Among the OSA subjects, the median apnea- hypopnea index was 23.3/h (15.8/h, 40.0/h interquartile range), indicative of moderate severity. Mean minimal oxygen saturation during the polysomnogram was 88 ± 5% in the OC group and 76 ± 10% in the OSA group.
Vascular reactivity in the macrocirculation
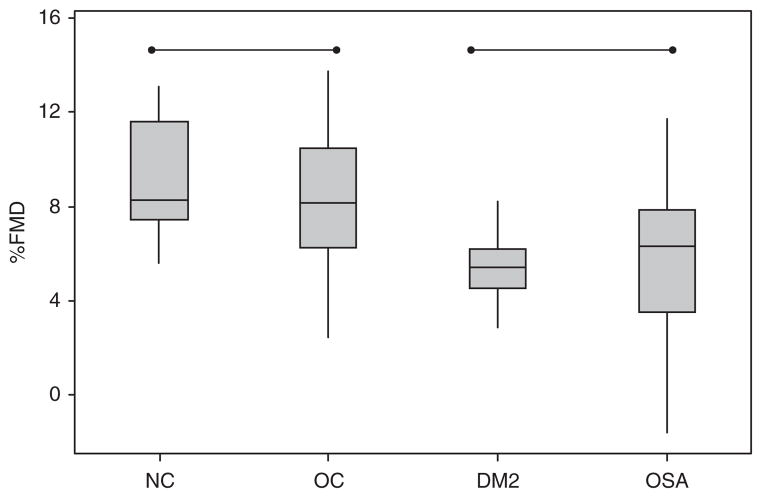
The baseline brachial artery diameter for each group is as follows: NC group (2.88 ± 0.48 mm), OC group (3.13 ± 0.63 mm), type 2 diabetes group (3.88 ± 0.73 mm Hg), and OSA group (3.49 ± 0.78 mm Hg, P < 0.001 for ANOVA). Figure 1 shows the differences in % change in brachial artery diameter in response to FMD. The OSA group and DM group had impaired FMD as compared to both the normal-weight and OC groups (P < 0.001, post hoc Fischer test). To adjust for covariates, in a step-forward linear regression inclusive of age, BMI, gender, and baseline brachial artery diameter, only baseline brachial artery diameter was significant in predicting the % change in FMD after ischemic hyperemia (%FMD). Table 2 depicts a multiple linear regression of baseline brachial artery diameter, OC, DM, and OSA status. When referenced to the NC group, DM status, OSA status, and baseline brachial artery diameter negatively predicted % FMD (Table 2).
Figure 1.
Endothelial function, as measured by flow-mediated dilation (FMD) of the brachial artery, is impaired in diabetes mellitus and obstructive sleep apnea subjects compared to normal-weight control and obese control subjects (P < 0.001). The box encompasses the 25–75% quartiles, and the median is represented by the horizontal line within the box. The whiskers extend to the highest and lowest values within the higher and lower limits, respectively. %FMD, % increase from baseline of the brachial artery after forearm ischemia; NC, normal-weight control; OC, obese control; DM2, type 2 diabetes mellitus; OSA, obstructive sleep apnea.
Table 2.
Multiple linear regression of %FMD (% change in brachial artery diameter after flow-mediated dilation), a measure of endothelial function in the brachial artery
| Predictor | β Coefficient | P value |
|---|---|---|
| Obese control | −0.43 | 0.670 |
| Type 2 diabetes | −2.31 | 0.022 |
| OSA | −2.45 | 0.015 |
| Baseline brachial artery diameter (mm) | −3.75 | 0.000 |
Groups were referenced to the normal-weight controls. Although baseline brachial artery diameter was the major predictor of %FMD, type 2 diabetes and OSA status negatively predicted %FMD as well. For this model, R2 = 23.5%.
OSA, obstructive sleep apnea.
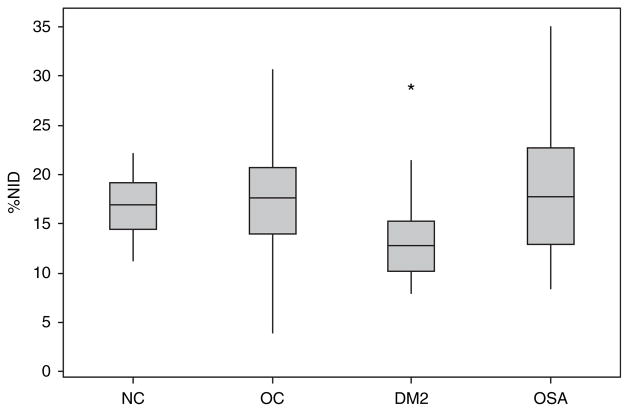
Figure 2 shows the % change in brachial artery diameter after administration of systemic nitroglycerin (%NID), a measure of endothelial-independent vasodilation. Compared to both the control and OSA groups, the DM group had impaired arterial smooth muscle dilation after systemic nitroglycerin (P < 0.001, post hoc Fischer’s). In a step-forward linear regression model inclusive of OC, DM status, OSA status, age, BMI, sex, and baseline brachial arterial diameter, only baseline brachial arterial diameter negatively predicted %NID. A multiple linear regression model inclusive of baseline brachial arterial diameter, OC, DM, and OSA groups is depicted in Table 3.
Figure 2.
Arterial smooth muscle function, as measured by dilation of the brachial artery in response to systemic nitroglycerin, is decreased in the diabetes mellitus group compared to the normal-weight control, obese control, and obstructive sleep apnea subjects (P < 0.001). The box encompasses the 25–75% quartiles, and the median is represented by the horizontal line within the box. The whiskers extend to the highest and lowest values within the higher and lower limits, respectively. %NID, % increase from baseline of the brachial artery in response to systemic nitroglycerin; NC, normal-weight control; OC, obese control; DM2, diabetes mellitus type 2; OSA, obstructive sleep apnea.
Table 3.
Multiple linear regression model of %NID (% change of the brachial artery after systemic nitroglycerin, a measure of endothelial-independent vasodilation)
| Predictor | β Coefficient | P value |
|---|---|---|
| Obese control | 0.87 | 0.388 |
| Type 2 diabetes | −0.61 | 0.542 |
| OSA | 1.89 | 0.061 |
| Baseline brachial artery diameter | −5.03 | 0.000 |
Groups were referenced to the normal-weight control group. Baseline brachial artery diameter was the only statistically significant predictor of %NID. For this model, R2 = 29.7%.
OSA, obstructive sleep apnea.
Vascular reactivity in the microcirculation
Baseline flow before iontophoresis of Ach for each group is as follows: NC (1.01 ± 0.25 V), OC (0.71 ± 0.21 V), DM (1.11 ± 0.30 V), and OSA (0.66 ± 0.19 V, P < 0.001, post hoc Fischer’s). Baseline flow before iontophoresis of SNp was similar (data not shown).
Both the OC and the obese OSA groups had higher % change in laser Doppler flowmetry after iontophoresis of Ach (endothelial-dependent) compared to the NC and DM groups (80.1 ± 43.2%, 66.9 ± 39.4% vs. 45.4 ± 24.3%, 45.8 ± 29.2%, respectively, P < 0.001 post hoc Fischer’s test). Similarly, both the control and obese OSA groups had higher % change in laser Doppler flowmetry after iontophoresis of SNp ( endothelial-independent) compared to the normal-weight and DM groups (68.0 ± 35.0%, 59.5 ± 37.4% vs. 37.1 ± 19.1%, 28.5 ± 28.3%, respectively, P < 0.001 post hoc Fischer’s test). In a step-forward linear regression model inclusive of OC, DM status, OSA status, age, BMI, sex, and baseline flow, baseline flow (β coefficient = −3.49, P = 0.001) and age (β coefficient = −2.98, P = 0.003) negatively predicted %Ach. Female sex positively predicted %Ach (β coefficient = 1.73, P = 0.09). In a multiple linear regression model inclusive of baseline flow, age, gender, OC, DM, and OSA groups, baseline flow was the only significant predictor of %Ach (β coefficient = −2.8, P = 0.006). For this model, R2 = 0.23. Age also negatively predicted %Ach, but was not statistically significant (β coefficient = −1.85, P = 0.07).
To determine predictors of %SNp, regression models were built. In a step-forward linear regression model inclusive of OC, DM status, OSA status, age, BMI, sex, and baseline flow, baseline flow (β coefficient = −7.27, P = 0.001) negatively predicted %SNp. Compared to the reference NC group, the OC group (β coefficient = 2.98, P = 0.02) positively predicted % SNp. In a multiple linear regression model inclusive of baseline flow, OC, type 2 diabetes, and OSA groups, baseline flow was the only significant predictor of %SNp (β coefficient = −5.32, P < 0.001). For this model, R2 = 0.36.
Metabolic syndrome and OSA
Because there is an increased prevalence of metabolic syndrome in OSA, the prevalence of metabolic syndrome in our obese OSA group was determined using the AHA guidelines. Thirteen of the 38 subjects with OSA met criteria for metabolic syndrome. None of the OC subjects were diagnosed with metabolic syndrome. %FMD of OSA group with metabolic syndrome was similar to the OSA without metabolic syndrome groups (5.1% (3.9%, 7.4%), 6.4% (2.9%, 8.0%), respectively). For %NID, OSA group with metabolic syndrome had similar values to the OSA group without metabolic syndrome (16.2% (11.9%, 22.6%) vs. 18.1% (14.8, 23.1), respectively).
DISCUSSION
The main novel finding in this study is that the effect of OSA on endothelial function is similar to that of DM. To our knowledge, this is the first study to compare endothelial function between OSA and type 2 diabetes. Furthermore, OSA impairs function of the endothelium, but does not seem to impair smooth muscle function or microcirculatory vascular function. These findings represent an important addition to the literature and will be critical to the design of subsequent interventional diabetes studies.
Although considerable literature exists on the link between OSA and DM (23), the majority of these efforts have focused on whether OSA predisposes to DM or whether DM predisposes to OSA. Furthermore, there has been controversy as to whether treatment of OSA improves glycemic control in DM. However, to our knowledge, there has been minimal focus on the interactions of these diseases from the standpoint of vascular complications.
We believe based on underlying mechanisms that OSA and DM may contribute to vascular complications through similar pathways. For example, oxidative stress is known to occur in both DM (through glucose oxidation, nonenzymatic glycation of proteins, and the subsequent oxidative degradation of glycated proteins) and OSA (through hypoxemia and reoxygenation) (24). In addition, autonomic abnormalities including hypovagotonia have been reported with both OSA and DM. Thus, there exists compelling rationale that OSA and DM may interact from the standpoint of vascular complications. In theory, OSA and DM could interact in the development of vascular complications, e.g., synergistically worsen vascular complications, or could potentially offset one another through ceiling effects. Given that the magnitude of the vascular impairment was similar in OSA and DM, and the effects were independent in multivariate analyses, we believe that this interaction deserves further attention. Because many patients with DM also have undiagnosed OSA, there may indeed be ceiling effects limiting the degree of vascular impairment seen in these patients.
Although substantial literature has shown improvements in diabetes complications with various interventions, several recent reports suggest a plateau in benefits with glycemic control particularly from the standpoint of macrovascular complications. Thus, some investigators have suggested that focus should be redirected to other conditions that may be contributing to the vascular impairment. Based on our results, we believe that OSA may be such a condition given the magnitude of the associated impairment in macrovacular function. The lack of effect of OSA on the microcirculation suggests that other therapeutic pathways should be considered for minimizing these associated complications in patients with both OSA and DM. The differential effects of DM and OSA on vascular smooth muscle vs. the endothelium further emphasizes the requirement for the consideration of parallel therapeutic pathways to minimize overall vascular risk.
Our study has considerable strengths including its novelty, a relatively large sample size for a physiological study, and for providing a compelling rationale for further research in the OSA/ DM link. However, we acknowledge a number of limitations. First, although none of our DM patients had known OSA, our DM patients did not undergo in-laboratory polysomnography and it is possible if not likely that many of these patients had undiagnosed OSA. Thus, subsequent research will be required to determine whether OSA plus DM has more vascular impairment than DM or OSA alone. However, given the finding that OSA in isolation had similar vascular risk to that of DM (with or without OSA), our findings are likely biased toward the null hypothesis because, if anything, the undiagnosed OSA in DM would be expected to worsen macrovascular function. It is also unlikely that the impairment in endothelial function seen in the DM group was solely due to undiagnosed concomitant OSA because a myriad of prior studies has shown endothelial dysfunction in DM in both animal and human research. Furthermore, the variability in %FMD in our OSA group speaks to the heterogeneity in OSA phenotypes. Therefore, the absence or presence of OSA alone cannot account for the persistent finding of impaired endothelial function in DM. We can only say that the degree of endothelial dysfunction seen in a group of OSA subjects is similar to a group of DM subjects. Although we believe our findings are important, our conclusions are clearly limited to the populations studied. Second, one could argue that because we were performing a physiological study, we measured only surrogate outcome measures rather than hard outcomes like myocardial infarction and stroke. We accept this limitation but would argue that physiological research should be performed to establish rationale for large observational population-based studies and interventional randomized clinical trials. Third, our research was cross-sectional, and therefore, our design does not allow us to separate correlation from causation. We accept this limitation but again would argue that such research is required before interventional or longitudinal research can be effectively designed. For example, based on our results to date, an interventional study to assess the impact of OSA therapy on microcirculatory function is probably not justified. Finally, the populations studied were not perfectly matched on the several baseline characteristics. We adjusted for covariates in our multiple regression analyses, and although baseline brachial artery size was a predictor of both endothelial-dependent and -independent vasodilation, the effect of OSA status was still apparent in FMD. Conversely, in the skin microcirculation, OSA status did not predict either endothelial-dependent or -independent vasodilation when adjusted for baseline flowmetry, perhaps as a result of incomplete matching between groups. We also found that the presence of metabolic syndrome in the OSA group (13/38) did not alter our results, but acknowledge a small sample size for this comparison. Similarly, our groups were not perfectly matched on aging, but we found only a weak correction of FMD with aging (r = 0.28 in univariate analysis), with no significant aging effect in multivariate analysis. Moreover, the fact that our OSA patients were somewhat younger than our DM subjects speaks to the importance of the OSA effect on vascular function because an older OSA group may have had even worse vascular function. Despite these limitations, we believe that our research provides a useful addition to the literature and that meaningful conclusions can be drawn. Future research in the interacting vascular complications of DM and OSA populations may provide new therapeutic options for afflicted patients.
In conclusion, we found that OSA impairs endothelial function in the brachial artery to a similar degree as DM does. However, OSA does not appear to affect brachial artery smooth muscle function or skin microcirculatory function. Treatment of OSA in patients with concomitant DM, therefore, may be a potential therapeutic option to improve macro-, but not microvascular outcomes.
Acknowledgments
A.M. has received funding from an Established Investigator Award of the AHA as well as K24 HL 093218, R01 HL090897-01, R01 HL085188-01, and R01 HL73146. A.V. is funded by R01-HL075678, R01 DK076937, and R01 DK076937. S.Y.-Y. received National Sleep Foundation’s Pickwick Fellowship. S.R. is funded by a grant from the American Sleep Medicine Foundation.
Footnotes
DISCLOSURE
A.M. has received consulting and/or research funding from Philips, Sepracor, NMT, Apnex, Itamar, Pfizer, SGS, Medtronic, Ethicon, Cephalon, and SHC. The other authors declared no conflict of interest.
References
- 1.Malhotra A, Hillman D. Obesity and the lung: 3. Obesity, respiration and intensive care. Thorax. 2008;63:925–931. doi: 10.1136/thx.2007.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Lewis CE, McTigue KM, Burke LE, et al. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation. 2009;119:3263–3271. doi: 10.1161/CIRCULATIONAHA.109.192574. [DOI] [PubMed] [Google Scholar]
- 5.McTigue K, Hess R, Bryce CL, et al. Perception of “healthy” body weight by patients with diabetes. Diabetes Care. 2006;29:695–697. doi: 10.2337/diacare.29.03.06.dc05-2459. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahangdale S, Yeh SY, Malhotra A, Veves A. Therapeutic interventions and oxidative stress in diabetes. Front Biosci. 2009;14:192–209. doi: 10.2741/3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Einhorn D, Stewart DA, Erman MK, et al. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract. 2007;13:355–362. doi: 10.4158/EP.13.4.355. [DOI] [PubMed] [Google Scholar]
- 9.Foster GD, Sanders MH, Millman R, et al. Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecube A, Sampol G, Lloberes P, et al. Diabetes is an independent risk factor for severe nocturnal hypoxemia in obese patients. A case-control study. PLoS ONE. 2009;4:e4692. doi: 10.1371/journal.pone.0004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra A, Loscalzo J. Sleep and cardiovascular disease: an overview. Prog Cardiovasc Dis. 2009;51:279–284. doi: 10.1016/j.pcad.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 14.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 15.Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottini P, Redolfi S, Dottorini ML, Tantucci C. Autonomic neuropathy increases the risk of obstructive sleep apnea in obese diabetics. Respiration. 2008;75:265–271. doi: 10.1159/000100556. [DOI] [PubMed] [Google Scholar]
- 18.Caballero AE, Arora S, Saouaf R, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 19.Kohler M, Craig S, Nicoll D, et al. Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. Am J Respir Crit Care Med. 2008;178:984–988. doi: 10.1164/rccm.200805-717OC. [DOI] [PubMed] [Google Scholar]
- 20.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 21.Veves A, Saouaf R, Donaghue VM, et al. Aerobic exercise capacity remains normal despite impaired endothelial function in the micro- and macrocirculation of physically active IDDM patients. Diabetes. 1997;46:1846–1852. doi: 10.2337/diab.46.11.1846. [DOI] [PubMed] [Google Scholar]
- 22.AASM. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in adults. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 23.Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:3878–3884. doi: 10.1210/jc.2008-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavie L. Oxidative stress–a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–312. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]