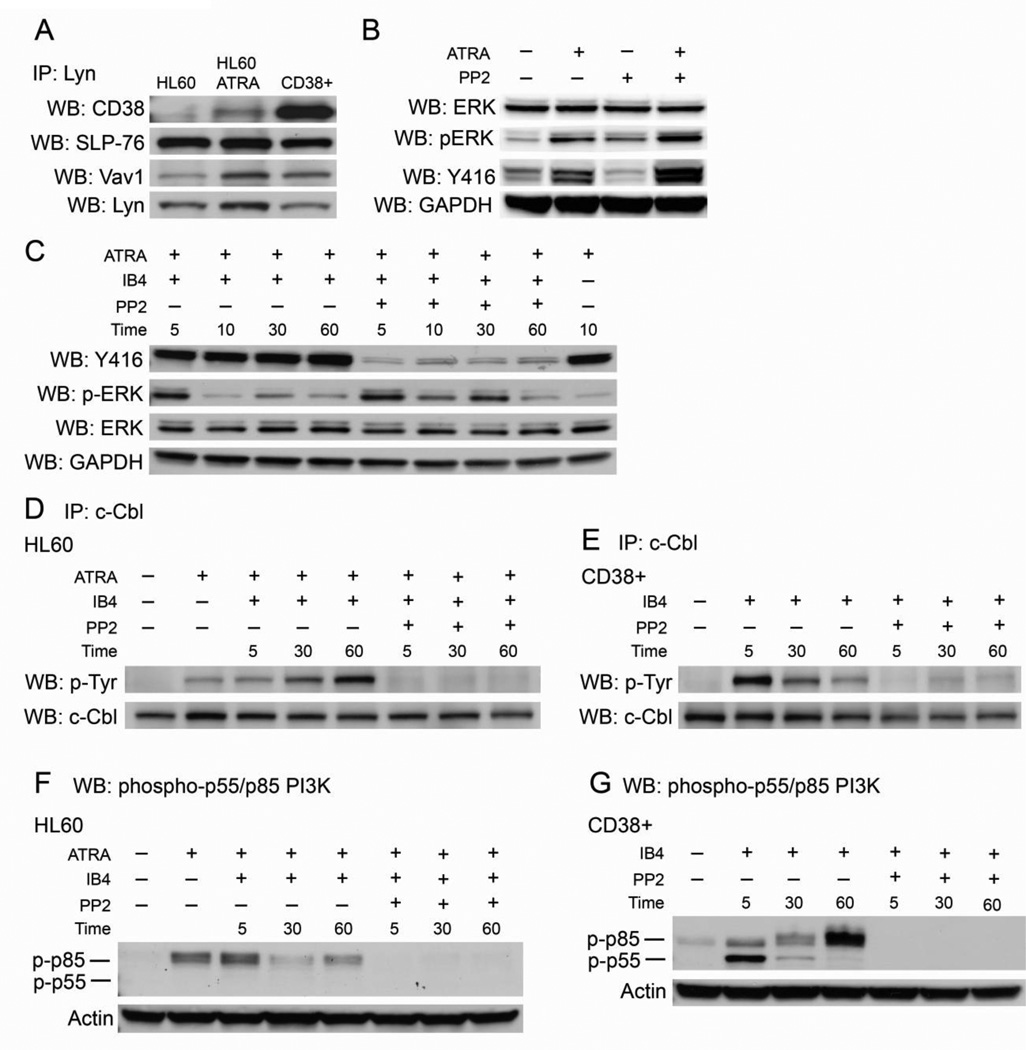
Figure 3. Lyn binds CD38, SLP-76, and Vav1 and its kinase activity regulates CD38 signaling.
A: Immunoprecipiation shows Lyn interaction with CD38, SLP-76, and Vav1 in HL-60 cells with and without 48 hours of ATRA treatment, and in untreated CD38+ cells. The IP:Lyn/WB:Lyn blot shows protein loading. B: Western blots for SFK/Lyn kinase basal activity in untreated, ATRA-treated, PP2-treated, and co-treated cells. All treatments lasted 48 hours. C: Western blots for SFK/Lyn kinase activity in ATRA-treated HL-60 cells using a pan-Y416 that detects active site phosphorylation in all SFK members. Cells were treated with ATRA for 48 hours, incubated with PP2 for one hour, and then stimulated by IB4 for the indicated time points. p-ERK blot shows PP2 did not significantly affect ERK phosphorylation. Total ERK and GAPDH show protein loading. D: Immunoprecipitation of c-Cbl shows tyrosine phosphorylation with indicated treatments in HL-60 cells. Cells treated with ATRA were cultured for 48 hours, cells treated with PP2 were exposed to the inhibitor for one hour, and cells were stimulated by IB4 for the indicated time points E: c-Cbl tyrosine phosphorylation in CD38+ transfectants in the absence of ATRA treated as indicated with PP2 for one hour and then stimulated with IB4. F: Western blot for phospho-p55/p85 PI3K in HL-60 cells treated as indicated. G: Western blot for phospho-p55/p85 PI3K in CD38+ transfectants treated as indicated. Actin shows protein loading in pY-p55/p85 PI3K blots.