Abstract
Despite modern antiretroviral therapy, HIV-associated sensory neuropathy affects over 50% of HIV patients. The clinical expression of HIV neuropathy is highly variable: many individuals report few symptoms, but about half report distal neuropathic pain (DNP), making it one of the most prevalent, disabling and treatment-resistant complications of HIV disease. The presence and intensity of pain is not fully explained by the degree of peripheral nerve damage, making it unclear why some patients do, and others do not, report pain. To better understand central nervous system contributions to HIV DNP, we performed a cross-sectional analysis of structural magnetic resonance imaging (MRI) volumes in 241 HIV-infected participants from an observational multi-site cohort study at five US sites (CNS HIV Antiretroviral Treatment Effects Research Study, CHARTER). The association between DNP and the structural imaging outcomes was investigated using both linear and nonlinear (Gaussian Kernel support vector) multivariable regression, controlling for key demographic and clinical variables. Severity of DNP symptoms was correlated with smaller total cerebral cortical gray matter volume (R = −0.24; p = 0.004). Understanding the mechanisms for this association between smaller total cortical volumes and DNP may provide insight into HIV DNP chronicity and treatment-resistance.
Keywords: HIV Distal Neuropathic Pain, Structural MRI, Cortical Volume
INTRODUCTION
Persistent pain now affects so many individuals with HIV infection that it has recently been termed an “evolving epidemic” (Wiebe et al 2011). HIV-associated distal neuropathic pain (DNP) is one of the most prevalent neurologic complications of HIV infection in the era of combination antiretroviral therapy (CART), affecting approximately 20% of patients (Ellis et al 2010). HIV DNP is typically refractory to current chronic pain therapies (Simpson et al 2008; Verma et al 2004) and is associated with unemployment, impairment in activities of daily living, and significantly diminished quality of life (Ellis et al 2010). Despite the prevalence, persistence, and impact of HIV DNP, little is known of its neurobiological underpinnings.
Over half of HIV-infected patients have sensory neuropathy by physical examination or nerve conduction studies (Ellis et al 2010; Robinson-Papp et al 2010). About 40% of them report chronic DNP, while the remainder report only numbness or paresthesiae or no symptoms at all (Ellis et al 2010; Robinson-Papp et al 2010). Most research on HIV DNP mechanisms has focused on the direct effects of HIV or antiretroviral drugs on peripheral nerves (eg, exposure to dideoxynucleoside reverse transcriptase inhibitors such as stavudine or didanosine) and on clinical risk factors for neuropathy (age, height, and lower CD4 nadir) (Ellis et al 2010). This research suggests the intensity of DNP is not fully explained by the extent of HIV damage to peripheral nerve fibers (Cherry et al 2005; Dorsey et al 2006; Herrmann et al 2004; Skopelitis et al 2007) or by clinical risk factors (Ellis et al 2008, 2010), leaving it unclear why some neuropathy patients experience DNP and others do not.
Central nervous system pathways may exert a major influence on the clinical expression of peripherally-induced pain (Apkarian et al 2005; Lee et al 2010; Ossipov et al 2010) and contribute to the transition from acute to chronic pain states (Borsook et al 2011; Baliki et al 2012). Chronic pain has been associated with reduced cortical brain volumes (Apkarian et al 2004; Baliki et al 2011; May et al 2008; May et al 2009; May et al 2011; Smallwood et al 2013). A better understanding of brain structure therefore might help explain the variable DNP presentations for patients with HIV-associated sensory neuropathy. Despite its potential usefulness, no reports of brain imaging to investigate HIV DNP have been published. Application of neuroimaging in this arena may be potentially confounded by neuromedical and neuropsychiatric co-morbidities such as HIV itself, traumatic brain injury (Lin et al 2011), depression (Grieve et al 2013; Truong et al 2013), and abuse of drugs, such as alcohol (Geibprasert et al 2010), inhalants (Borne et al 2005; Geibprasert et al 2010), and methamphetamine (Jernigan et al 2005), which are associated with changes in regional brain volumes. In particular, HIV distal neuropathic pain is associated with depression (Lucey et al 2011; Keltner et al 2012) and depression can contribute to changes in brain structure (Grieve et al 2013; Truong et al 2013). Thus, neuroimaging research must be conducted within the context of a comprehensive evaluation if results are to be interpretable.
We performed a cross-sectional analysis of regional brain volumes measured by structural magnetic resonance imaging (MRI) in 241 HIV-infected individuals, 66 with DNP and 175 without DNP, participating in an observational multi-site cohort study. Since total cortical gray matter is reduced in volume in persons with chronic back pain (Apkarian et al 2004, Baliki et al 2011), we hypothesized that more severe DNP symptoms would be associated with smaller total cortical gray matter volume. Because participants underwent concurrent standardized medical, neurological, neuropsychological, and psychiatric evaluation, we could statistically control for confounding conditions.
METHODS
Participants
Of 1,556 HIV patients at five US academic medical centers participating in the CNS (Central Nervous System) HIV Anti-Retroviral Therapy Effects Research Study (CHARTER), 241 underwent structural magnetic resonance imaging (MRI) (Jernigan et al 2011). The sites performing MRI included: Johns Hopkins University (Baltimore, MD, n=47); Mount Sinai School of Medicine (New York, NY, n=48); University of California at San Diego (San Diego, CA, n=70); University of Texas Medical Branch (Galveston, TX, n=46); and University of Washington (Seattle, WA, n=30). The 241 participants in the MRI substudy met criteria for and agreed to undergo an MRI (Jernigan et al 2011). Data reported here are from their first MRI at their second CHARTER visit, which occurred six months after the baseline CHARTER visit.
Standard Protocol Approvals and Patient Consents
These procedures were approved by the Human Subjects Protection Committees of each participating institution. Written informed consent was obtained from all study participants as part of enrollment into the peripheral neuropathy and MRI substudy.
Measures and Assessments
Diagnosis of HIV Sensory Neuropathy
Using procedures described in detail in a prior publication (Ellis et al 2010), the diagnosis of HIV Sensory Neuropathy (HIV-SN) was rendered by physicians and nurses trained in neurological AIDS disorders based on a standardized, neurological examination evaluating HIV-associated sensory neuropathy signs, including diminished ability to recognize vibration and reduced sharp-dull discrimination in the feet and toes or reduced ankle reflexes. We defined at least one sign of neuropathy bilaterally as evidence of HIV sensory neuropathy. Of the 241 participants in this substudy, 102 had no signs of neuropathy, 69 had one sign, and 70 had two or more signs. Of the 102 participants with no signs of neuropathy, 9 (9%) had DNP (defined below); of the 69 participants with one sign of neuropathy, 20 (29%) had DNP; and of the 70 participants with two or more signs of neuropathy, 37 (53%) had DNP.
Diagnosis of HIV Distal Neuropathic Pain
As described previously (Ellis et al 2010; Robinson-Papp et al 2010), HIV DNP was defined as a specific pattern of bilateral burning, aching, or shooting pain in a distal gradient in the lower extremities. Recognizing that this specific pattern of pain may occur in small fiber-predominant neuropathies in which clinical exam abnormalities are sometimes absent due to the relative paucity of large fiber involvement, we included in the diagnosis of DNP those cases that did not have abnormal clinical exam findings. Indeed, some cases of HIV-SN have been shown to manifest predominantly small fiber involvement. Study clinicians classified DNP into five categories of severity: none, slight (occasional, fleeting), mild (frequent), moderate (frequent, disabling), and severe (constant, daily, disabling, requiring analgesic medication or other treatment). In the statistical model these categories were treated as continuous variables: 0=none (175 participants), 1=slight (18 participants), 2=mild (22 participants), 3=moderate (10 participants), and 4=severe (16 participants). In the assessments of DNP severity, additional characteristics were elicited including the continuity as well as its impact on daily activities and the need for analgesic medications.
Medical Evaluation
Clinicians conducting the neurological examination also performed semi-structured interviews and standardized examinations to ascertain HIV disease status, HIV treatment history, psychotropic medications, and pain treatments. Venipuncture and lumbar puncture were performed. Plasma and cerebrospinal fluid (CSF) HIV concentration were determined by reverse transcription polymerase chain reaction ultrasensitive assay (nominal lower quantitation limit, 50 copies/mL [Amplicor; Roche Diagnostic Systems, Indianapolis, Indiana]) and assayed for co-infection with hepatitis C virus (Hepatitis C Virus Antibody Assay [HCV]; LabCorp, Burlington, North Carolina). Current peripheral blood CD4+ T cell concentration was measured by flow cytometry. We also obtained historical indicators of HIV disease such as the lowest ever CD4 lymphocyte count (CD4 nadir) by chart review or participant recall, since CD4 nadir < 200 is associated with worse neuromedical outcomes. Immune recovery was calculated as the difference between the nadir CD4 and current measured CD4 levels.
An extensive treatment history queried for prior and current combination antiretroviral drugs (CART) and patients were categorized as currently using, past history of, or never used CART. We assessed past and current use of a protease inhibitor or a non-nucleoside reverse-transcriptase inhibitor; total number of protease inhibitors being used and for exposure to dideoxynucleoside reverse transcriptase inhibitiors (didanosine, stavudine, and zalcitabine) since these agents cause neuropathy. Finally, we surveyed for current pain treatment with opioids, tricyclic and other antidepressants, and/or anticonvulsants.
Neurocognitive Impairment: Global Deficit Score
HIV is associated with brain atrophy and cognitive impairment (Herrmann et al 2004; Jernigan et al 2011). Neuropsychological function was assessed with a battery of tests that yields a Global Deficit Score (GDS) ranging from 0 = no to 9 = severe impairment and calculated from less than expected performance after adjustments for age, education, and ethnicity/race on each test. Global deficit scores are highly effective in identifying neuropsychological impairment in HIV patients when an adequate numbers of tests are used (Carey et al 2004).
Traumatic Brain Injury
Since brain trauma can impact regional brain volumes, history of traumatic brain injury (TBI) was obtained using a standardized medical history (Lin et al 2011). A traumatic brain injury score (ranging from 0 to 4) was determined for each patient based on history obtained from a structured neuromedical and a neuropsychological interview (Lin et al 2011). Of the 241 participants in this study, 54 provided an inconsistent history in the two interviews, so their traumatic brain injury status was rated as “unknown”. Of the remaining 187, 45 had no history of TBI (TBI score = 0); 128 had a history of concussion or head injury with 0–30 minutes loss of consciousness (TBI score = 1); 12 had a history of loss of consciousness greater than 30 minutes (TBI score = 2); 1 had a history of brain trauma complicated by neurological deficits lasting greater than 2 weeks (TBI score = 3); and 1 had a history of brain trauma with permanent residual neurological and motor impairments (TBI score = 4).
Psychiatric and Substance Misuse Disorders
Because psychiatric and substance use disorders may be associated with neuroimaging and cognitive abnormalities, current (e.g., within the last 30 days) and lifetime history of major depression and psychoactive substance use disorders were assessed by the computer-assisted Composite International Diagnostic Interview (Wittchen et al 1991). History of abuse and dependence was collected for alcohol, cannabis, cocaine, hallucinogens, inhalants, methamphetamine, opioids, and sedatives.
The Beck Depression Inventory-second edition (BDI-II) (Beck 1996), which has been validated for HIV populations (Lipps et al 2010), was used to assess depressive symptoms over the past two weeks. The BDI-II consists of 21 questions, each having four graded statements (labeled 0 to 3) ordered to show increasing severity of depressive symptoms. Four categories/levels of depression severity are derived based upon the total numerical score: 0–13 = Minimal; 14–19 = Mild; 20–28 = Moderate; 29–63 = Severe.
Structural Imaging Analysis Methods
As described previously (Jernigan et al 2011), structural MRI volumes were acquired on 1.5 Tesla GE scanners at the five participating sites. Four series were acquired for multi-channel tissue segmentation and anatomy: Series 1 and 2 were coronal acquisitions with section thickness=2.0 mm, FOV 24 cm, matrix size 256×256: 2D T2-weighted fast spin echo (FSE) with TR=5700ms, TE=90ms, ETL=16; and 2D proton density (PD) weighted FSE with TR=3700ms, TE=17ms, ETL=4. Series 3 and 4 were sagittal acquisitions with section thickness=1.3mm, FOV 24 cm, matrix size 256×256×124: 3D T1-weighted SPGR with TR=20ms, TE=6ms, flip angle=30; and 3D PD-weighted SPGR sequence with TR=20ms, TE=6ms, flip angle=5.
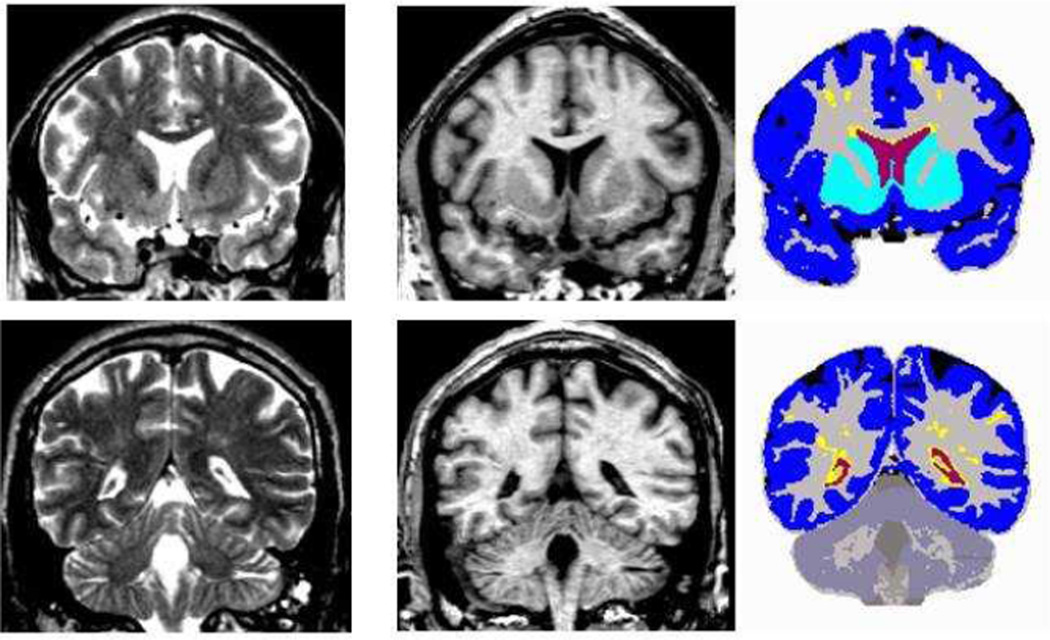
The standard multi-channel morphometric analysis method employed by the CHARTER study (Jernigan et al 2011) provided measures of cortical and subcortical gray matter; total cerebral white matter and abnormal white matter; and ventricular and sulcal CSF (Figure 1). This approach includes image inspection for motion and other artifacts; bias correction; co-registration of MRI volumes; re-slicing in a standard space; selection of tissue samples in gray matter, white matter, and CSF by trained neuro-anatomist masked to other patient information; removal of non-brain voxels; automated tissue segmentation; abnormal white matter designation; and anatomical segmentation. Information from all four MRI series described above is leveraged for the multi-channel approach. Tissue segmentation is based on successive linear regressions on the tissue samples to separate CSF from brain and then to separate gray from white matter. In this approach, abnormal white matter regions are defined as voxels within the total cerebral white matter that have signal values that fall in (or beyond) the distribution estimated from the gray matter sample (i.e., outside the range of normal white matter, for example, hyperintense regions on T2), and these voxels are segmented as “gray”. Anatomists processed the tissue-segmented images manually to separate the cerebellum from the cerebrum, the ventricles from sulcal fluid in the subarachnoid space, and cortical from subcortical gray matter within the cerebrum. Cortical gray matter volumes include neocortex and the hippocampus and amygdala; subcortical gray matter volumes include the caudate, putamen, nucleus accumbens, thalamus, and some substantia nigra and ventral diencephalic regions.
Figure 1.
Multi-channel morphometry. Coronal T2 (left), T1 (middle), and segmented (right) images for anterior (top) and posterior (bottom) sections. Volumes include: ventricular (red) and subarachnoid (black) CSF, abnormal (yellow) and total (grey + yellow) white matter, cortical (blue) and subcortical (turquoise) gray matter
Statistical Methods
The association between DNP and the structural imaging variables was investigated using univariable and multivariable regression to predict DNP severity rating (from 0 = none to 4 = severe). DNP was treated as a continuous variable in the statistical analyses. All structural volumes were log transformed to symmetrize the distributions and stabilize the variances. Supratentorial cranial vault volume and scanner were included as covariates in both univariable and multivariable analyses to control for individual differences in head size as well as differences in scanners across sites (Fennema-Notestine et al 2007; Jernigan et al 2011).
In the multivariable analyses, the model is subject to a model selection step, whereby only potential confounders that are significant at the 0.20 level are included in the final multivariable model. In addition, in the multivariable analyses we controlled for variables that may impact regional brain volumes based on previous work (Borne et al 2005; Geibprasert et al 2010; Jernigan et al 2005; Jernigan et al 2011; Grieve et al 2013; Truong et al 2013). These included socio-demographic characteristics (age, gender, race/ethnicity, education); clinical characteristics and comorbidities (plasma and cerebrospinal fluid HIV RNA levels - detectable versus undetectable); number of signs of neuropathy (0 signs, 1 sign, 2 or more signs); current and nadir CD4+ T-cell counts; hepatitis C serology (positive versus negative); neurocognitive impairment (Global Deficit Score); treatment history (current antiretroviral treatment - yes or no, antiretroviral regimen type, number of protease inhibitors, antiretroviral dideoxynucleoside reverse transcriptase inhibitors use - current and past use as well as current and past total exposure); history of substance abuse and dependence (yes or no for alcohol, cannabis, cocaine, hallucinogens, inhalants, methamphetamine, opiates, and sedatives); depression characteristics (yes or no for diagnosis of major depression - current and past, as well as total BDI-II score); and current use of analgesic medication (opioids, tricyclic antidepressants/serotonin norepinephrine reuptake inhibitors, anticonvulsants).
In the multivariable models, a subject was excluded if data for that subject was missing from any explanatory variable included in the model. Traumatic brain injury was not included in our multivariable analysis since over 25% of the subjects were missing TBI scores: 54 subjects had unknown TBI scores due to inconsistent TBI reports; however, we did complete a multivariable analysis investigating the association of DNP with log cortical volumes where TBI was included in the model selection process.
For both the univariable and multivariable analyses we calculated the proportional difference in mean brain volume per one unit of DNP severity (and 95% CI) as determined by exponentiating the regression coefficient of DNP in the log-volume analysis; the Cohen’s d effect size associated with DNP and 95% confidence intervals; the corresponding p-value from the t-test of regression with Bonferonni correction for multiple comparisons – each p-value was multiplied by six since six different analyses were performed for each regional brain volume; the total R-squared model fit; and the partial R-squared model fit for DNP. The partial R-squared model fit for DNP between each regional brain volume vs DNP, R2DNP, was calculated using the formula (Cohen et al 2003), R2DNP = (R2DNP,Other - R2Other)/(1 - R2Other), where R2DNP,Other is the R-squared model fit for the multivariable regression between each regional brain volume and confounding variables (significant at the p=0.20 level) included in the final multivariable model as well as DNP, while R2Other is R-squared model fit for the multivariable regression between the regional brain volume and these same confounding variables without DNP. All tests were two-sided, and statistical significance was declared at the 0.05 level. All computations were done using JMP (SAS Institute, Cary, North Carolina, U.S.) and R statistical platforms (R Foundation, Vienna, Austria).
In follow-up analyses, using logistic regression we investigated how the odds of pain decreased for increasing adjusted log cortical volumes where the adjusted log cortical volumes were divided into quartiles. The adjusted log cortical volumes were the residuals from a linear regression using log cortical volumes as the dependent variable and explanatory variables from the multivariable analyses that were significant at the p=0.20 level as the independent variables.
In addition, the correlation was calculated between log cortical volumes versus severity of DNP using a scatter-plot of adjusted log cortical volume residuals plotted vs adjusted DNP residuals; the adjusted log cortical volumes and the adjusted DNP were again calculated using explanatory variables from the multivariable log cortical volume model that were significant at the p=0.20 level.
RESULTS
The demographic and clinical characteristics of the study participants (Table 1) show that typical patients were middle-aged men of Caucasian and African-American ethnicity who had AIDS and were currently on antiretrovirals, with good immune recovery and fair virologic control.
TABLE 1.
Demographic and clinical characteristics of participants, both with and without distal neuropathic pain (DNP).
| No. of subjects | 241 |
| Age, mean (SD) | 43.8 (7.9) |
| Education yrs, mean (SD) | 12.8 (2.5) |
| Male, No. (%) | 192 (80) |
| Race/Ethnicity, No. (%) | |
| Caucasian | 93 (39) |
| African American | 115 (48) |
| Hispanic | 27 (11) |
| Other | 6 (2) |
| Hepatitis C Virus seropositive, No. (%) | 68 (28) |
| AIDS, No. (%) | 152 (63) |
| Global Deficit Score, mean (SD) | 0.46 (0.46) |
| CD4, No. (%) | |
| Current < 200; Nadir < 200 | 34 (14) |
| Current >= 200, Nadir < 200 | 106 (44) |
| Current >= 200, Nadir >= 200 | 98 (41) |
| CART Use, No. (%) | |
| Current | 177 (73) |
| Past | 37 (15) |
| Never | 27 (11) |
| Plasma HIV RNA Detectable, No. (%) | 122 (51) |
| ON CART, No. (%) | 61 (35) |
| OFF CART, No. (%) | 61 (97) |
| Cerebrospinal Fluid HIV RNA Detectable, No (%) | 69 (32) |
| ON CART, No. (%) | 24 (16) |
| OFF CART, No. (%) | 45 (76) |
| Current Antiretroviral Regimen, No. (%) | |
| None | 64 (26) |
| PI-based * | 96 (54) |
| NNRTI-based * | 61 (34) |
| PI+NNRTI-based * | 10 (6) |
| Other | 11 (6) |
| D-drug* use, past or current, No. (%) | 132 (55) |
| Lifetime Major Depressive Disorder**, No. (%) | 52 (22) |
| Current Major Depressive Disorder**, No. (%) | 27 (11) |
| Beck Depression Inventory II, Mean (SD) | 12.3 (11.0) |
| Neuropathic pain rating, No. (%) | |
| Slight | 18 (7) |
| Mild | 22 (9) |
| Moderate | 10 (4) |
| Severe | 16 (6) |
| Zero signs of neuropathy, No (%) | 102 (42) |
| One sign of neuropathy | 69 (29) |
| Two signs of neuropathy | 70 (29) |
| In those with no signs of neuropathy, No (%) | |
| HIV distal neuropathic pain | 9 (9) |
| No HIV distal neuropathic pain | 93 (91) |
| In those with one sign of neuropathy, No (%) | |
| HIV distal neuropathic pain | 20 (29) |
| No HIV distal neuropathic pain | 49 (71) |
| In those with two or more signs of neuropathy, No (%) | |
| HIV distal neuropathic pain | 37 (53) |
| No HIV distal neuropathic pain | 33 (47) |
| Current pain treatment, No. (%) | |
| Opioids | 42 (17) |
| TCA/SNRI | 23 (10) |
| Anticonvulsant | 23 (10) |
| Traumatic Brain Injury, No. (%) | |
| Inconsistent data | 54 (22) |
| TBI rating = 0 | 45 (19) |
| TBI rating = 1 | 128 (53) |
| TBI rating = 2 | 12 (5) |
| TBI rating = 3 | 1 (.004) |
| TBI rating = 4 | 1 (.004) |
PI = Protease Inhibitor; NNRTI = Non-nucleoside reverse-transcriptase inhibitor; D-drug = dideoxynucleoside reverse transcriptase inhibitiors; CART = Combination antiretroviral therapy; TCA= Tricyclic antidepressant; SNRI = Serotonin norepinephrine reuptake inhibitor.
Current Major Depressive Disorder = Meets DSM-IV criteria for a major depressive episode within the last 30 days
As a point of reference for conceptualizing the MRI results, Figure 1 illustrates typical T1 and T2 slices images used in the multi-channel morphometric segmentation of regional brain volumes, as well as ventricular and subarachnoid CSF, abnormal and total white matter, and total cortical and subcortical gray matter.
Univariable and multivariable analyses of the association of DNP with the structural imaging outcomes (Table 2) reveal that worse (higher) clinician-assessed DNP rating was associated with lower total cortical gray matter volume. In the univariable analyses worse DNP was associated with larger ventricular CSF volume. In both the univariable and multivariable analyses the DNP rating was not associated with subcortical gray matter volumes, abnormal white matter volume, white matter total volume, or sulcal CSF volume.
TABLE 2.
Association between DNP and structural imaging outcomes in univariable and multivariable regression analyses.
| UNIVARIABLE ANALYSES | MULTIVARIABLE ANALYSES | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume (log transformed) |
Numb. Subj. |
Percent Change Proportional Difference in Mean Brain Volume Per One Unit of DNP (95% CI) |
Effect Size (95% CI) |
p-value* | R2 | Partial R2 | Numb. Subj. |
Percent Change Proportional Difference in Mean Brain Volume Per One Unit of DNP (95% CI) |
Effect Size (95% CI) |
p- value* |
R2 | Partial R2 |
| Cortical Gray Matter | 241 | −1.35 (−0.06,−2.12) | −0.19 (−0.30,−0.08) | 0.005 | 0.74 | 0.048 | 213 | −1.27 (−1.98,−0.56) | −0.21 (−0.34,−0.09) | 0.004 | 0.83 | 0.059 |
| Subcortical Gray Matter | 241 | −0.70 (−1.66, 0.25) | −0.08 (−0.19, 0.03) | 0.905 | 0.32 | 0.009 | 238 | −0.23 (−1.16, 0.71) | −0.03 (−0.14, 0.09) | 1 | 0.41 | 0.001 |
| Abnormal White Matter | 241 | 0.89 (−2.71, 4.63) | 0.03 (−0.08, 0.14) | 1 | 0.27 | 0.001 | 236 | 0.93(−2.54, 4.54) | 0.03 (−0.09, 0.15) | 1 | 0.42 | 0.001 |
| Total White Matter | 241 | −0.15 (−1.22, 0.93) | −0.02 (−0.13, 0.09) | 1 | 0.57 | 0.000 | 241 | 0.10 (−1.12, 0.94) | −0.01 (−0.12, 0.10) | 1 | 0.63 | 0.000 |
| Ventricular CSF | 241 | 7.80 (−2.32,13.99) | 0.15 ( 0.04, 0.26) | 0.034 | 0.24 | 0.033 | 241 | 7.35 ( 1.81,13.19) | 0.16 ( 0.04, 0.28) | 0.056 | 0.42 | 0.031 |
| Sulcal CSF | 241 | 7.20 ( 1.02, 13.76) | 0.13 ( 0.02, 0.24) | 0.136 | 0.20 | 0.022 | 241 | 4.18 (−1.54,10.23) | 0.08 (−0.03, 0.19) | 0.941 | 0.33 | 0.009 |
Numb. Subj. = Number subjects without missing data
p-value* = p-value corrected for multiple comparison
DNP was present, but neuropathy signs absent, in only 9 of 66 subjects with DNP (14%). Excluding these 9 cases did not alter the relationship between severity of DNP symptoms and smaller total cerebral cortical gray matter volume (R2 = .82; p = 0.004).
Note that TBI was not included in the multivariable model as described in the statistical methods section. When TBI is included as a variable in the model selection step for the multivariable analysis investigating the association of DNP with the log cortical volumes, the calculated variables in Table 2 are not significantly different for all variables: percent change in proportional difference in mean brain volume per one unit of DNP, effect size, p-value, the total R-squared model fit, and the partial R-squared model fit for DNP.
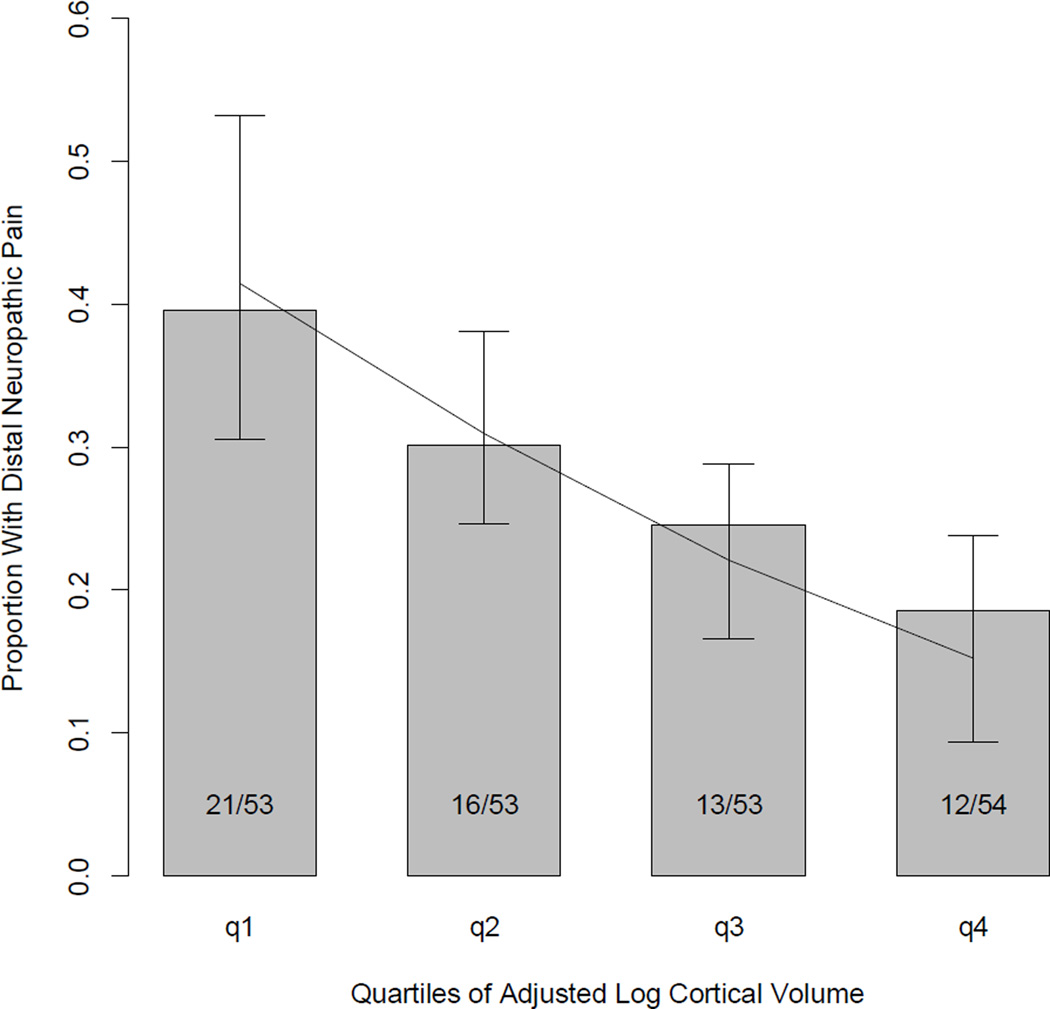
The odds of pain decreased by a factor of OR=1.42, 95% CI=(1.08, 1.87) for each increasing quartile of adjusted log cortical brain size. The proportions of frequency of distal neuropathic pain for each quartile of adjusted log cortical volume are plotted in Figure 2.
Figure 2.
Proportion of subjects with distal neuropathic pain per quartile of adjusted log cortical volume. The line represents the fitted values (and 95% confidence intervals) from the logistic regression model. Quartile #1 represents the 53 of the 213 research subjects with the smallest log cortical volumes while quartile #4 represents the 54 subjects of the 213 research subjects with the largest log cortical volumes. The fractions (i.e. 21/53) inside each bar of the bar graph represent the proportion of the quartile of subjects with distal neuropathic pain.
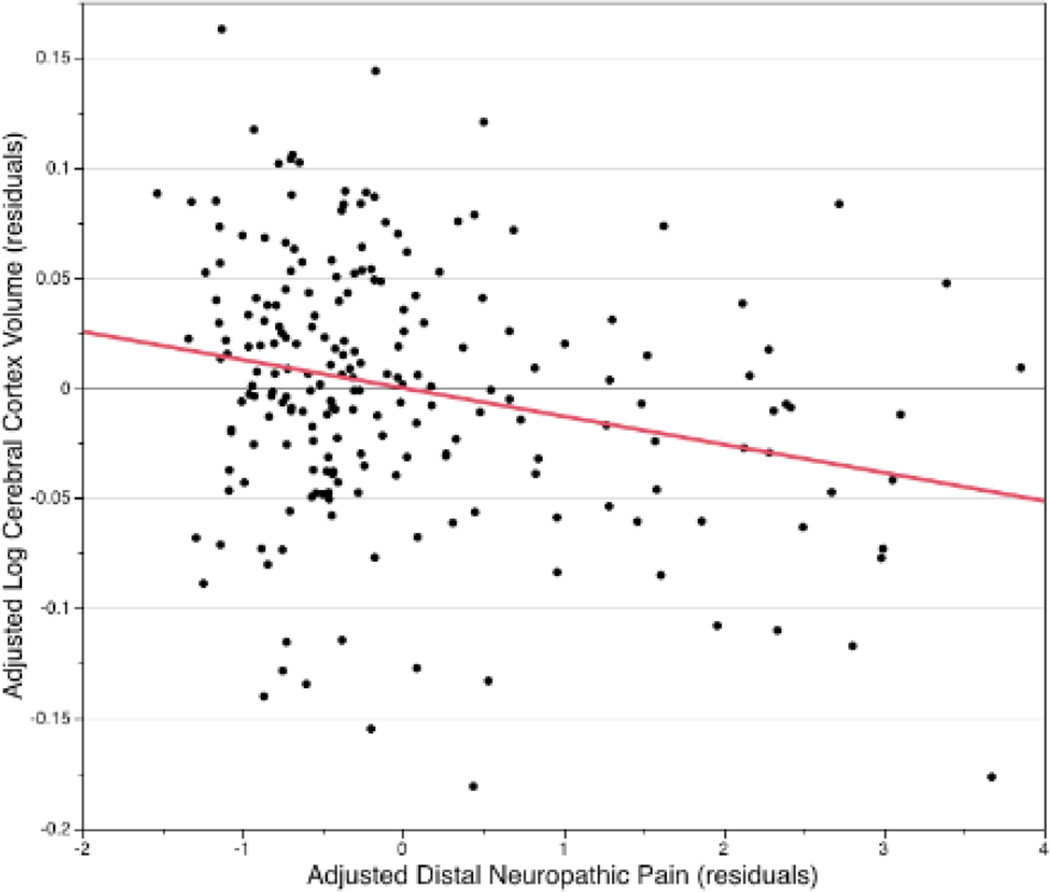
We calculated the linear correlation coefficient between adjusted log cortical volume residuals plotted vs adjusted DNP residuals (R=-0.24 p=0.0003), see figure 3. The R squared fit = 0.059 for this linear fit agrees with the multivariable log cortical volume partial R squared fit for DNP in Table 2.
Figure 3.
A Partial correlation scatterplot (plot of partial residuals) shows the relationship between DNP and log-transformed cortical gray matter volume, after adjusting for all other variables in the multivariable regression model. The line represents the linear best fit for this data; correlation coefficient and p-value are described in the text.
Given previous investigation of the impact of HIV on brain structure (Jernigan et al 2011), it is notable that DNP severity was correlated with age (r = 0.22, p = 0.006) but was not significantly correlated with CD4 nadir, dideoxynucleoside reverse transcriptase inhibitors use (current or past), or global deficit score.
The explanatory variables used for each multivariable linear regression model of the association between DNP and log brain volumes are shown in supplementary table, S1. In this table, the definition (type and range), number of subjects with missing data for each variable, and the p-values (uncorrected for multiple comparisons) less than 0.20 (based on linear regression) are listed for each explanatory variable in this table. Supplementary table S2 gives variable ranking results based on nonlinear regression.
DISCUSSION
Consistent with our hypothesis and with previously reported findings in HIV-uninfected persons, more severe DNP was associated with smaller volumes of total cerebral cortical gray matter in HIV-infected patients. To better understand associations between HIV pain and brain volume, we statistically controlled for several HIV disease-related factors and non-HIV characteristics (eg, substance abuse and depression) that could contribute to this relationship.
A distal, sensory predominant, axonal neuropathy can be demonstrated by clinical examination, by nerve conduction studies, by quantitative sensory testing, and skin biopsy analysis of epidermal nerve fiber density in more than half of HIV-infected individuals on long-term antiretroviral therapy (Robinson-Papp et al 2010; Simpson et al 2006). Chronic DNP and disability are reported by some patients, yet many remain asymptomatic or experience only non-painful symptoms such as paresthesiae and numbness. This raises the question of why the clinical phenotype (DNP) varies between individuals who have similar underlying signs of peripheral neuropathy. Differences in central nervous system pain processing are one possible explanation for the variation in expression of DNP for HIV patients.
Possible explanations for the observed correlations between increased DNP severity and smaller total cortical gray matter volumes include: 1) DNP causes reduced cortical volumes, 2) reduced cortical volumes cause DNP, 3) both reduced cortical volumes and DNP are causally linked to a third, confounding variable, and 4) statistical coincidence. Alternatively, cortical atrophy may be a surrogate marker -- rather than a direct cause itself -- for a pathological process that directly alters pain perception. For example, cortical volume loss may track with neuronal damage in circuits responsible for the central processing of emotional and cognitive information about pain.
The repeated observation that successful treatment of chronic pain can normalize regional brain volumes suggests that the direction of causality may be from chronic pain to reductions in regional brain volumes (Gwilym et al 2010; Rodriguez-Raecke et al 2009; Seminowicz et al 2011; May et al 2011;). Moreover, multiple chronic pain syndromes such as headache (Schmitz et al 2008) and back pain (Apkarian et al 2004; Baliki et al 2011), are associated with smaller regional cerebral cortical gray volumes (May et al 2011; Smallwood et al 2013). Our result that total cortical gray matter is smaller for patients with HIV DNP is consistent with Apkarian and Baliki’s observations that total cortical gray matter is smaller in patients with chronic back pain (Apkarian et al 2004; Baliki et al 2011). Interestingly, total cortical gray matter volume is not reduced in patients with complex regional pain syndrome and knee osteoarthritis (Baliki et al 2011). It has been suggested that different chronic pain syndromes exhibit unique structural brain imaging anatomical ‘brain signatures’ (Baliki et al 2011).
The effects of DNP appear to be regionally different and unrelated to biomarkers of HIV-infection. HIV is associated with decreased basal ganglia volume, increased abnormal white matter volume, and to a lesser extent decreased cortical volumes; so HIV may have contributed to brain atrophy in our sample (Jernigan et al 2011; Paul et al 2002). However, HIV most often is associated with alterations of subcortical gray and white matter and DNP was not associated with these regional volumes. Moreover, effects of DNP were independent of HIV-related factors such as viral load and CD4+ T-cell count that were accounted for in the multivariable model.
On the other hand, cortical atrophy may contribute to the development of chronic pain. For example, the prevalence of chronic pain is increased in several CNS diseases that cause cortical atrophy (Borsook et al 2011): including Parkinson’s disease (40–60%), Alzheimer disease (57%), multiple sclerosis (50–86%), and traumatic brain injury (57%). In addition, chronic pain is associated with changes in CNS function, structure, and chemistry, which are thought to contribute to the centralization of chronic pain (Borsook et al 2011).
This study has several strengths and limitations. It is based in a large prospective, diverse cohort drawn from multiple US sites and provided rigorous standardized assessment of both brain structure and neuromedical variables. Limitations include its cross-sectional design, which leaves us unable to explore possible cause and effect relationships between cortical volumes and HIV DNP. We examined global brain volumes available from the CHARTER study, but have not explored associations of cortical regions with DNP. Our cohort was a convenience sample of patients enrolled in HIV care at academic clinics, rather than from a random sample of the HIV patients in care.
Given the high prevalence and disability caused by HIV-associated DNP, the need to improve understanding of the effects and mechanisms of DNP, and the need to identify therapeutic targets, additional research is warranted to assess whether any regional specificity within the cortex is associated with HIV DNP.
Supplementary Material
Acknowledgments
This research was supported by awards N01 MH22005, R25-MH081482, HHSN271201000027C, R01 MH079752 and P30 MH0625 from the National Institutes of Health. Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government. Each author John R. Keltner, Christine Fennema-Notestine, Florin Vaida, Dongzhe Wang, Donald R. Franklin, Robert H. Dworkin, Chelsea Sanders, J. Allen McCutchan, Sarah L. Archibald, David J. Miller, George Kesidis, Clint Cushman, Sung Min Kim, Ian Abramson, Michael J. Taylor, Rebecca J. Theilmann, Michelle D. Julaton, Randy J. Notestine, Stephanie Corkran, Mariana Cherner, Nichole A. Duarte, Terry Alexander, Jessica Robinson-Papp, Benjamin B. Gelman, David M. Simpson, Ann C. Collier, Christina M. Marra, Susan Morgello, Greg Brown, Igor Grant, J. Hampton Atkinson, Terry L. Jernigan and Ronald J. Ellis does not have a financial relationship with the National Institutes of Health.
Footnotes
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: J. Allen McCutchan, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Terry Alexander, R.N.; Laboratory, Pharmacology and Immunology Component: Scott Letendre, M.D. (P.I.), Edmund Capparelli, Pharm.D.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Matthew Dawson; Virology Component: David Smith, M.D. (P.I.); Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry L., Jernigan, Ph.D., Michael J. Taylor, Ph.D., Rebecca J. Theilmann, Ph.D., John Hesselink, M.D.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D.; Protocol Coordinating Component: Thomas D. Marcotte, Ph.D. (P.I.); Johns Hopkins University Site: Justin McArthur (P.I.), Mary Smith; Mount Sinai School of Medicine Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P., Cheuk Tang, Ph.D., and Thomas Naidich, M.D.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Will Toperoff, N.P.; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Kenneth Maravilla, MD, KC Stegbauer, Ph.D., Trudy Jones, M.N., A.R.N.P.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N., Gregory Chaljub, M.D.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
Disclosures:
The authors have no conflicts of interest to disclose.
REFERENCES
- Aksu Y, Miller DJ, Kesidis G, Yang QX. Margin-Maximizing Feature Elimination Methods for Linear and Nonlinear Kernel SVMs. IEEE Trans. Neural Networks. 2010 May;21(5):701–717. doi: 10.1109/TNN.2010.2041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of Neuroscience. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLos One. 2011;Vol 6(Issue 10):1–13. doi: 10.1371/journal.pone.0026010. e26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nature Nueuroscience. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Beck depression inventory: manual. 2 ed. The Psychological Corporation; 1996. [Google Scholar]
- Borne J, Riascos R, Cuellar H, Vargas D, Rojas R. Neuroimaging in drug and substance abuse part II: opioids and solvent. Top Magn Reson Imaging. 2005;16:239–245. doi: 10.1097/01.rmr.0000192154.34563.6b. [DOI] [PubMed] [Google Scholar]
- Borsook D. Neurological diseases and pain. Brain. 2011;135:320–344. doi: 10.1093/brain/awr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. HNRC Group: Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology. 2005;65:1778–1781. doi: 10.1212/01.wnl.0000187119.33075.41. [DOI] [PubMed] [Google Scholar]
- Chang CC, Lin CJ. LIBSVM: a library for support vector machines. ACM Transactions on Intelligent Systems and Technology. 2011;2(3):27:1–27:27. Software available at http://www.csie.ntu.edu.tw/~cjlin/libsvm. [Google Scholar]
- Cohen J, Cohen P, West SG. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. London: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Dorsey SG, Morton PG. HIV peripheral neuropathy: pathophysiology and clinical implications. AACN Clin Issues. 2006;17:30–36. doi: 10.1097/00044067-200601000-00004. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Marquie-Beck J, Delaney P, Alexander T, Clifford DB, McArthur JC, Simpson DM, Ake C, Collier AC, Gelman BB, McCutchan JA, Morgello S, Grant I. CHARTER Group: Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol. 2008;64:566–572. doi: 10.1002/ana.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. CHARTER Study Group: Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Archives of Neurology. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Gamst AC, Quinn BT, Pacheco J, Jernigan TL, Thal L, Buckner R, Killiany R, Blacker D, Dale AM, Fischl B, Dickerson B, Gollub RL. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5:235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- Geibprasert S, Gallucci M, Krings T. Alcohol-induced changes in the brain as assessed by MRI and CT. Eur Radiol. 2010;20:1492–1501. doi: 10.1007/s00330-009-1668-z. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage: Clinical. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62:2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- Herrmann DN, McDermott MP, Henderson D, Chen L, Akowuah K, Schifitto G. North East AIDS Dementia (NEAD) Consortium: Epidermal nerve fiber density, axonal swellings and QST as predictors of HIV distal sensory neuropathy. Muscle Nerve. 2004;29:420–427. doi: 10.1002/mus.10567. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I. CHARTER Group: Clinical Factors Related to Brain Structure in HIV: The CHARTER Study. Journal of Neurovirology. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JR, Vaida F, Ellis RJ, Moeller-Bertram T, Fitzsimmons C, Duarte NA, Robinson-Papp J, Dworkin RH, Clifford DB, McArthur JC, Simpson DM, Collier AC, Marra CM, Atkinson JH, Grant I. CHARTER Group: Health-related quality of life ‘well-being’ in HIV distal neuropathic pain is more strongly associated with depression severity than with pain intensity. Psychosomatics. 2012;53(4):380–386. doi: 10.1016/j.psym.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Tracey I. Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr Pain Headache Rep. 2010;14:124–131. doi: 10.1007/s11916-010-0103-0. [DOI] [PubMed] [Google Scholar]
- Lin K, Taylor MJ, Heaton R, Franklin D, Jernigan T, Fennema-Notestine C, McCutchan A, Atkinson JH, Ellis RJ, McArthur J, Morgello S, Simpson D, Collier AC, Marra C, Gelman B, Clifford D, Grant I. CHARTER group: Effects of traumatic brain injury on cognitive functioning and cerebral metabolites in HIV-infected individuals. Journal of Clinical and Experimental Neuropsychology. 2011;33:326–334. doi: 10.1080/13803395.2010.518140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipps GE, Lowe GA, De La Haye W, Longman-Mills S, Clarke TR, Barton EN, Bain B. Validation of the Beck Depression Inventory II in HIV-positive Patients. West Indian Med J. 2010;59:374. [PubMed] [Google Scholar]
- Lucey BP, Clifford DB, Creighton J, Edwards RR, McArthur JC, Haythornthwaite J. Relationship of depression and catastrophizing to pain, disability, and medication adherence in patients with HIV-associated sensory neuropathy. AIDS Care. 2011;23(8):921–928. doi: 10.1080/09540121.2010.543883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- May A. Morphing voxels: the hype around structural imaging of headache patients. Brain. 2009;132:1419–1425. doi: 10.1093/brain/awp116. [DOI] [PubMed] [Google Scholar]
- May A. Structural brain imaging: A window into chronic pain. Neuroscientist. 2011;17(2):209–220. doi: 10.1177/1073858410396220. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. The Journal of Clinical Investigation. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neuroscience & Biobehavioral Reviews. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Robinson-Papp J, Morgello S, Vaida F, Fitzsimons C, Simpson DM, Elliott KJ, Al-Lozi M, Gelman BB, Clifford D, Marra CM, McCutchan JA, Atkinson JH, Dworkin RH, Grant I, Ellis R. Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy. Pain. 2010;151:732–736. doi: 10.1016/j.pain.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. The Journal of Neuroscience. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, van Buchem MA. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48:1044–1055. doi: 10.1111/j.1526-4610.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, Marra CM, Diaz-Arrastia R, Shriver S, Milllar L, Clifford DB. ACTG A5117 Study Group: HIV neuropathyt natural history cohort study: Assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70:2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- Skopelitis E, Aroni K, Kontos AN, Konstantinou K, Kokotis P, Karandreas N, Kordossis T. Early detection of subclinical HIV sensory polyneuropathy using intraepidermal nerve fibre density quantification: association with HIV stage and surrogate markers. Int J STD AIDS. 2007;7:856–860. doi: 10.1258/095646207782717054. [DOI] [PubMed] [Google Scholar]
- Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural Brain Anomalies and Chronic Pain: A Quantitative Meta-Analysis of Gray Matter Volume. The Journal of Pain. 2013;14:663–675. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smola AJ, Schölkopf B. A tutorial on support vector regression. Statistics and computing. 2004;14(3):199–222. [Google Scholar]
- Truong W, Minuzzi L, Soares CN, Frey BN, Evans AC, MacQueen GM, Hall GBC. Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Research: Neuroimaging. 2013;214:204–211. doi: 10.1016/j.pscychresns.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Verma S, Estanislao L, Mintz L, Simpson D. Controlling Neuropathic Pain in HIV. Current Infectious Disease Reports. 2004;6:237–242. doi: 10.1007/s11908-004-0014-5. [DOI] [PubMed] [Google Scholar]
- Wiebe LA, Phillips TJ, Li JM, Allen JA, Shetty K. Pain in HIV: an evolving epidemic. J of Pain. 2011;12:619–624. doi: 10.1016/j.jpain.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI) Br J Psychiatry. 1991;159:645–653. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.