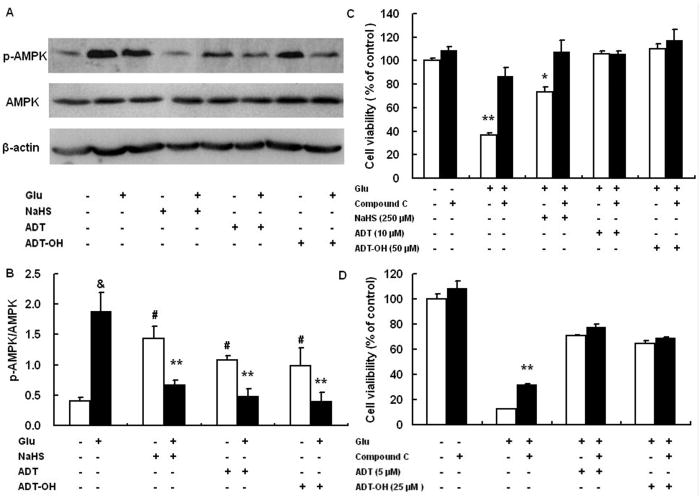
Figure 5.
AMPK activation contributed to cell death induced by glutamate and suppressing AMPK activation was a mechanism accounting for ADT and ADT-OH neuroprotection against oxidative glutamate toxicity. A: a representative image of western blot (upper panel) showed that, at 6 hours after glutamate exposure, HT22 cells exhibited increased phosphorylation (activation) of AMPK and that all hydrogen sulfide donors NaHS (250 μM), ADT-OH (50 μM) and ADT (10 μM) remarkably decreased glutamate-induced AMPK phosphorylation. B: The bar graph of western blot results. ** p<0.01 compared to cells treated with glutamate alone (n = 4); # p<0.05 compared to control cells without glutamate and hydrogen sulfide donor treatment; & p<0.01 compared to control cells without glutamate and hydrogen sulfide donor treatment. C: the AMPK inhibitor compound C robustly decreased HT22 death induced by glutamate oxidative stress and conferred additional neuroprotection in NaHS (250 μM) -treated HT22 cells following glutamate exposure, while it did not increased survival of HT22 cells treated with 50 μM ADT and 10 μM ADT-OH following glutamate exposure. ** p<0.01 and * p<0.05 compared with the corresponding black bars (cells treated with glutamate alone or cells with glutamate plus NaHS, n = 4). D: Compound C did not display synergetic effects on protecting HT22 cells from glutamate toxicity even when ADT and ADT-OH at the concentrations provided suboptimal protection (5 μM for ADT and 25 μM for ADT-OH, n=4). ** p<0.01 compared with glutamate-treated cells.