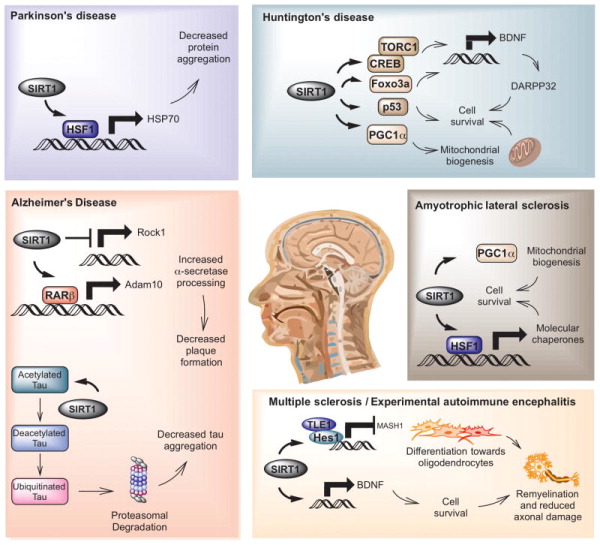
Figure 4. Major targets and mechanisms of SIRT1 in mouse models of neurodegenerative disease.
Alzheimer’s disease (highlighted in red) In Alzheimer’s disease, SIRT1 has been shown to promote non-amyloidogenic APP processing pathway by decreasing levels of ROCK1 kinase (Qin et al., 2006). SIRT1 may also target the retinoic acid receptor β, which activates ADAM10 to facilitate processing of APP along a non-amyloidogenic pathway (Donmez et al., 2010). SIRT1 has also been shown to directly deacetylate tau in several tauopathy models enabling ubiquitin ligases to promote clearance of this protein (Cohen et al., 2011; Min et al., 2010). Parkinson’s disease (highlighted in blue) SIRT1 has been shown to deacetylate HSF, which induces transcription of molecular chaperones that promote protein folding (Donmez et al., 2012; Raynes et al., 2012; Westerheide et al., 2009). In addition to its effects on the heat shock response, SIRT1 may also function to regulate autophagy and mitophagy, which may affect α-synuclein toxicity in the context of PD (Sampaio-Marques et al., 2012; Wu et al., 2011). There is also evidence that PGC1α may be a relevant target in mouse models of PD (Mudo et al., 2012). Huntington’s disease (highlighted in green) Several different molecular mechanisms account for the protective effect of SIRT1 overexpression against mutant huntingtin toxicity (Jeong et al., 2012; Jiang et al., 2012). Mutant huntingtin protein was found to inhibit the enzymatic activity of SIRT1 during HD pathogenesis. One proposed mechanism is that SIRT1 deacetylates TORC1, facilitating BDNF transcription through CREB (Jeong et al., 2012). An alternate explanation for the protective effect of SIRT1 in HD mice was that this protein might maintain TrkB signaling and DARPP32 levels as HD progresses. Foxo3a deacetylation was another SIRT1 target implicated in promoting cell survival in the HD models (Jiang et al., 2012). Amyotrophic lateral sclerosis (highlighted in beige) The proposed mechanism for SIRT1’s activity in Amyotrophic lateral sclerosis parallels one of the pathways observed in PD. SIRT1 has been shown to deacetylate HSF1, which increases transcription of molecular chaperones including HSP70 and HSP25 that help to maintain intracellular protein homeostasis, reducing toxicity to motor neurons (Han et al., 2012; Raynes et al., 2012; van Ham et al., 2008; Westerheide et al., 2009). It has also been shown to affect mitochondrial biogenesis in cell culture models of ALS and this may be due to deacetylation of PGC1α (Wang et al.) Multiple sclerosis (highlighted in orange) In experimental autoimmune encephalitis (EAE), the mouse model for multiple sclerosis (MS), the mechanism of protection in whole animal models has not been completely elucidated. In adult neuronal precursor cells (NPCs), SIRT1 binds Hes1 under oxidative conditions, inhibiting Mash1 transcription and driving NPCs toward astroglial differentiation (Prozorovski et al., 2008) and increasing the oligodendrocyte population may help remyelinate lesions, reduce axonal damage or decrease astrogliosis. An alternate approach has identified increased BDNF and NAMPT as possible targets to explain neuroprotection due to SIRT1 overexpression in EAE (Nimmagadda et al., 2013).