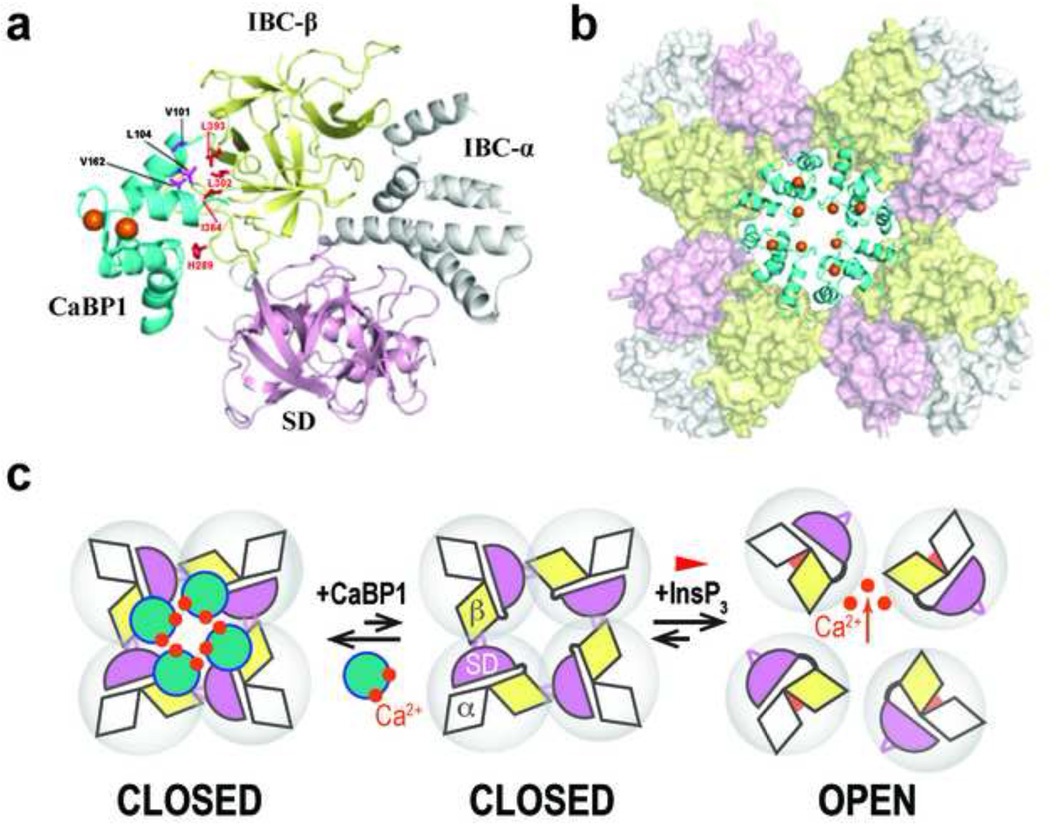
Figure 4. Structure of the InsP3R1-NT/CaBP1 complex and opposing effects of InsP3 and CaBP1 on intersubunit interactions.
a, Structure of the C-lobe of CaBP1 (CaBP1-C) bound to InsP3R1-NT in a 1:1 complex. NMR structural restraints were used to define contacts between InsP3R1-NT (SD, pink; IBC-β, yellow; IBC-α, grey) and the CaBP1-C (cyan, with Ca2+ atoms colored orange). Key residues at the binding interface are highlighted in magenta (CaBP1) and red (InsP3R1). b, Model for the tetrameric InsP3R1-NT/CaBP1-C complex (colored as in f) generated by superimposing the InsP3R1-NT crystal structure (Seo et al., 2012) onto the cryo-EM electron density map of InsP3R1 (Ludtke et al., 2011). c, Interactions between adjacent InsP3R1-NTs that are mediated by IBC-β (yellow) and the HS-loop of the SD (magenta) hold the tetrameric InsP3R1 in a closed state. InsP3 binding closes the clam-like IBC, disrupting these intersubunit interactions, and allowing the channel to open. CaBP1 clamps the intersubunit interactions associated with the closed-state, thereby inhibiting channel opening. The figures are reproduced from (Li et al., 2013).