Fig. 3.
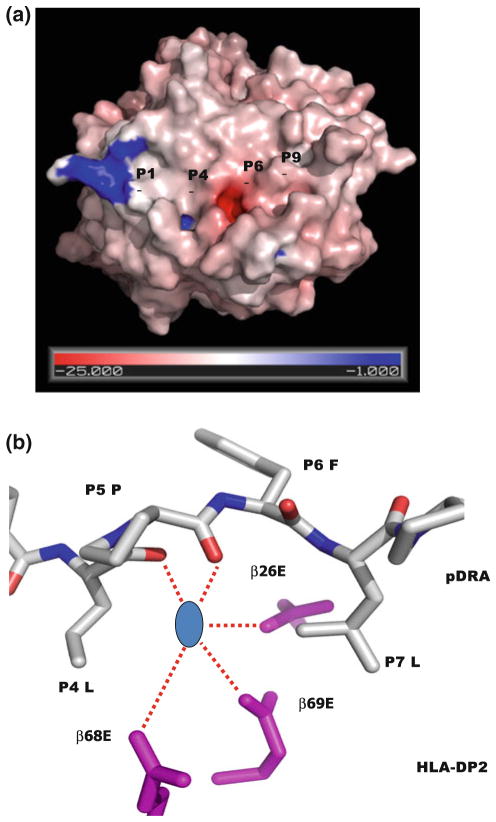
The unusual features of HLA-DP2 a The DP2/DRa peptide complex is viewed as Fig. 1. The water accessible surface of the DP2 molecule (without bound pDRA) is shown. The HLA-DP2 β-chain α-helix colored by the relative charge of the surface atoms (red, negative and blue, positive). The acidic pocket is located in the area between p5Pro and the DP2 β-chain α-helix. b A hypothetic Be compound is modeled in the acidic pocket. View of the acidic pocket looking down the peptide binding groove from the top. Wireframe representations of the side chains of β26Glu, β68Glu and β69Glu are colored in magenta. A wireframe representation of P4 to P6 of pDRA is shown with white carbon, red oxygen and blue nitrogen. Also, shown is a blue circle as a hypothetic Be compound