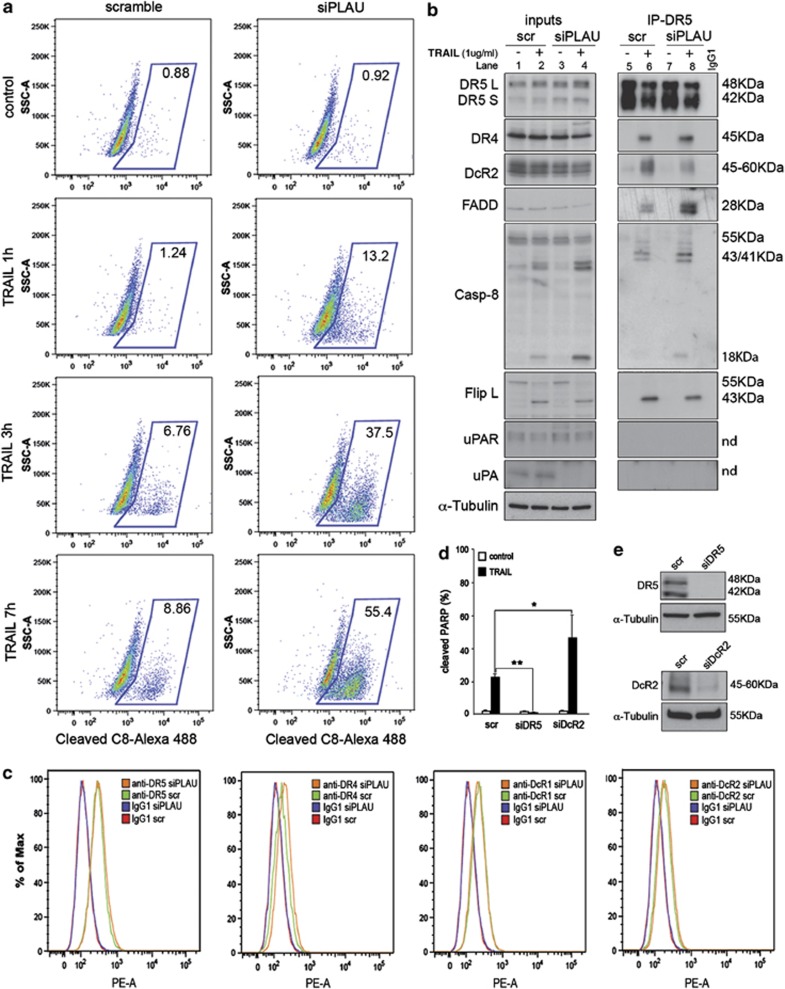
Figure 5.
Altered recruitment of inhibitory components to the DISC promotes caspase-8 activation in uPA knockdown cells. (a) Time-course analysis of procaspase-8 cleavage. BJELR cells were transfected either with pooled siRNAs targeting PLAU mRNA (‘siPLAU') or non-targeting scramble siRNAs (‘scramble'). Forty-eight hours after transfection, cell populations were either left untreated or challenged with 1 μg/ml TRAIL during 1, 3 or 7 h and the percentage of cells displaying cleaved caspase-8 (‘C8') was determined by flow cytometry. Images from one representative experiment out of three independent replicates are shown. Percentage of cleaved C8-positive cells is indicated. (b) DISC composition in uPA-depleted cells. BJELR cells were transfected either with pooled siRNAs targeting PLAU mRNA (‘siPLAU') or non-targeting scramble siRNAs (‘scr'), and 48 h after transfection, cell populations were either left untreated or challenged with TRAIL (1 μg/ml) during 30 min. Immunoprecipitation of Death Receptor 5 (IP-DR5) was performed and co-immunoprecipitation of cognate DISC components, uPA and uPAR, was analyzed by western blot analysis. Immunoprecipitation using isotypic IgG1 (‘IgG1') was used as background control. (c) Surface levels of TRAIL-Rs upon uPA knockdown. BJELR cells transfected either with pooled siRNAs targeting PLAU mRNA (‘siPLAU') or non-targeting scramble siRNAs (‘scr'). Forty-eight hours after transfection, surface levels of Death Receptor 5 (‘DR5'), Death Receptor 4 (‘DR4'), Decoy Receptor 1 (‘DcR1') and Decoy Receptor 2 (‘DcR2') were analyzed by flow cytometry. Isotypic IgG1 labeling was used as control for background fluorescence in scramble- (‘IgG1 scr') and siPLAU- (‘IgG1 siPLAU') transfected cells. (d) Antiapoptotic role of DcR2. BJELR cells were transfected either with pooled siRNA targeting Death Receptor 5 mRNA (‘siDR5'), Decoy Receptor 2 mRNA (‘siDcR2') or non-targeting scramble siRNAs (‘scr'), and 48 h after transfection, cell populations were either left untreated or challenged with TRAIL (1 μg/ml) during 3 h. Apoptosis was determined as the percentage of cells with positive labeling for cleaved PARP by flow cytometry. Histograms represent the mean±S.D. of three independent biological replicates. Statistical significance was calculated by applying two-tailed, unpaired Student's t-test, *P-value<0.05; **P-value<0.005. (e) Efficiency of DR5 and DcR2 knockdown at 48 h after transfection analyzed by western blot analysis. α-Tubulin, loading control