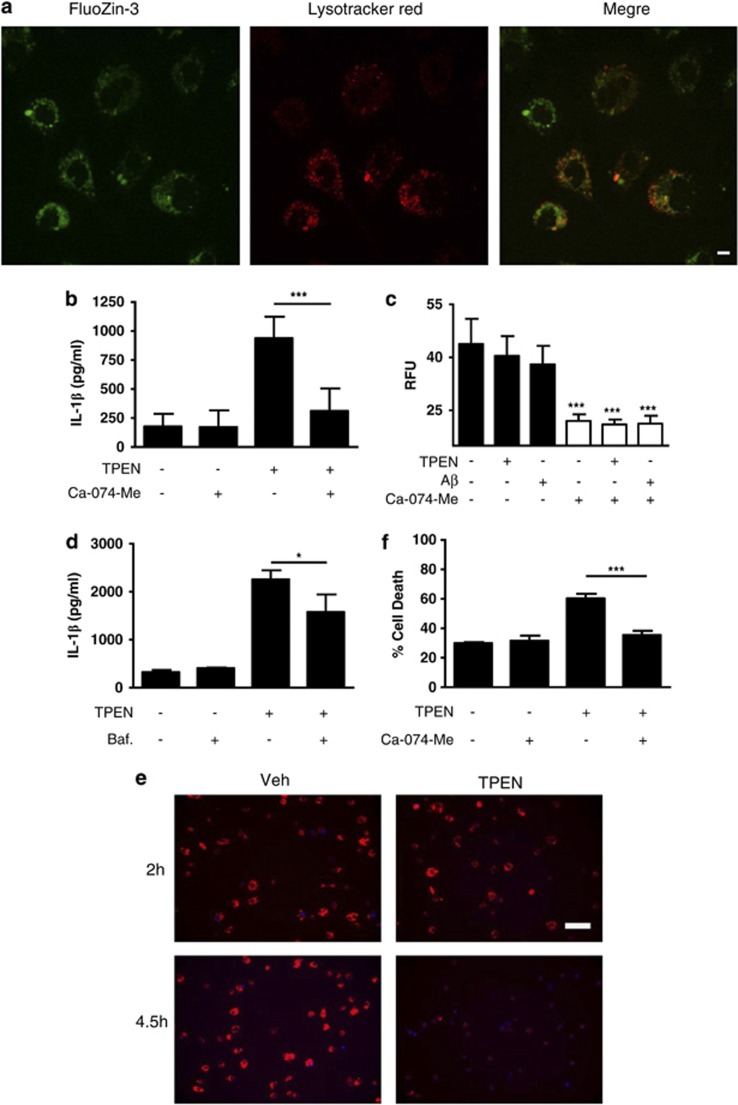
Figure 5.
TPEN-induced NLRP3 inflammasome activation depends upon a loss of lysosomal integrity. (a) LPS-primed (1 μg/ml, 2 h) peritoneal macrophages were loaded with FluoZin-3 and Lysotracker Red and imaged using a spinning disc confocal microscope. Scale bar represents 5 μm. (b) LPS-primed peritoneal macrophages were incubated with the cathepsin B inhibitor Ca-074-Me (80 μM) before incubation with TPEN (10 μM, 4 h) with IL-1β release measured by ELISA. (c) LPS-primed peritoneal macrophages were incubated with TPEN (10 μM, 4 h) or Aβ (5 μM, 4 h) after which cells were lysed in hypotonic lysis buffer. Cathepsin B/L-dependent cleavage of the fluorogenic substrate Z-Phe-Arg-AMC (40 μM) was measured by an increase in fluorescence (excitation 335 nm, emission 460 nm). The cathepsin B inhibitor Ca-074-Me (100 μM) was included as a control. (d) LPS-primed peritoneal macrophages were incubated with bafilomycin A (100 nM) before incubation with TPEN (10 μM, 4 h) with IL-1β release measured by ELISA. (e) LPS-primed (1 μg/ml, 2 h) peritoneal macrophages were loaded with Lysotracker Red and then incubated with DMSO (0.5%, Veh), or TPEN (10 μM) for 2 and 4.5 h. Snap shot images of live cells were taken using a BD Pathway Bioimager. Hoechst was included in the culture media allowing labelling of cells in which plasma membrane integrity was compromised. Scale bar=50 μm. (f) LPS-primed peritoneal macrophages were incubated with TPEN (10 μM, 6 h) plus and minus Ca-074-Me (100 μM) with cell death measured by release of LDH. All data are presented as the mean±S.D. from at least four separate experiments. All images are representative. ***P<0.001, *P<0.05