Abstract
The treatment of hepatitis C virus (HCV) infection with pegylated interferon alpha and ribavirin leads to a sustained virologic response in around 50% of patients with HCV genotype 1, 65% with HCV genotype 4, 75% with HCV genotype 3 and around 80% with HCV genotype 2. A better understanding of the HCV lifecycle has resulted in the development of several potential direct-acting antiviral drugs (DAAs) targeting viral proteins [NS3/4A protease inhibitors, NS5B nucleos(t)idic and non-nucleos(t)idic polymerase inhibitors, NS5A replication complex inhibitors]. This review summarizes the main clinical data for the combinations of oral DAAs. DAAs, either in combination with pegylated interferon alpha or in interferon-free regimens, have demonstrated a high level of antiviral efficacy and a generally well-tolerated safety profile in treatment-naïve patients and in prior nonresponders to pegylated interferon alpha/ribavirin. Oral combination of new DAAs is likely to become the standard of care for chronic HCV in treatment-naïve or treatment-experienced patients. However, most studies so far have included small numbers of ‘easy-to-treat’ patients with short post-treatment periods for defining the sustained virologic response. Extension of the number of treated patients (including ‘difficult-to-treat’ patients, i.e. patients infected with genotype 3, who failed to respond to first-generation protease inhibitors or with cirrhosis as well as immunocompromised patients) and of the post-treatment follow up in a real-life setting could significantly worsen the rate of recovery. In these ‘difficult-to-treat’ patients, the rate of virologic cure with new DAAs could be lower than expected and consequently interferons may be still necessary in combination with the new drugs.
Keywords: cirrhosis, combination therapy, direct-acting antiviral, hepatitis C virus, polymerase inhibitor, protease inhibitor, replication complex inhibitor
Introduction
Treatment of chronic hepatitis C with pegylated interferon alpha and ribavirin (PR) for a duration adapted according to the early virologic response results in effective and sustained viral suppression in less than 50% of patients infected with hepatitis C virus (HCV) genotype 1 (the commonest genotype in North America and Europe), in 65% with HCV genotype 4, 75% with genotype 3 and around 80% with genotype 2 [EASL, 2011]. A better understanding of the HCV lifecycle and the characterization of viral enzymes that are potential antiviral targets [Moradpour et al. 2007] have led to the development of a number of potential new direct-acting antiviral agents (DAAs) targeted against viral proteins [Buhler and Bartenschlager, 2012]. These include first-generation NS3/NS4A protease inhibitors, which mostly specifically target HCV genotype 1, and second-generation NS3/NS4A protease inhibitors, NS5B polymerase inhibitors, or NS5A inhibitors with a broader spectrum [Sarrazin et al. 2012; Yang et al. 2011]. Several non-DAAs, which could be associated with DAAs, are also under development, e.g. new interferons, cyclophilin inhibitors, monoclonal antibodies and vaccine therapy [Donnelly and Kotenko, 2010; Flisiak et al. 2007; Burioni et al. 2008].
Standard of care after 2011 with first-wave DAAs
Recent approval of the first-generation HCV NS3/4A protease inhibitors boceprevir and telaprevir, and their use in triple combinations with PR, has significantly improved sustained virologic response (SVR) rates by around 30% in genotype-1-infected treatment-naïve patients [Jacobson et al. 2011; Poordad et al. 2011] and those experienced with PR treatment [Bacon et al. 2011; Zeuzem et al. 2011a]. This was a major breakthrough but both agents have considerable side effects [Cacoub et al. 2012] (which add to those of PR), including severe skin rashes/pruritus and severe cutaneous adverse reaction (SCAR) (telaprevir), anal discomfort (telaprevir) and anaemia (telaprevir and boceprevir). In the real-life studies, cirrhosis decompensation and death related mainly to bacterial infections may occur in experienced Child A cirrhotic patients with albumin levels below 35 g/l and platelets count below 100,000/ml) [Hezode et al. 2012a]. In addition, telaprevir and boceprevir are dosed 2 and 3 times daily respectively, and carry a high pill burden (12 per day for boceprevir and 6 for telaprevir in addition of 4–7 for ribavirin) [Jacobson et al. 2011; Poordad et al. 2011; Bacon et al. 2011; Zeuzem et al. 2011a]. CYP3A4 and CYP3A5 metabolism requires drug adaptation and choice due to potential drug–drug interactions [Burger et al. 2013]. Finally, both telaprevir and boceprevir are approved only for genotype-1-infected patients even if an antiviral potency has been reported in genotype-2-infected patients (telaprevir) [Foster et al. 2011] and to a lesser extent in genotype-4-infected patients (telaprevir and boceprevir).
Thus, there remains a need for new therapeutic strategies with simplified oral dosing, broader efficacy across HCV genotypes, minimal side effects and improved tolerability profiles. Most of the new drugs (second-generation NS3/NS4A protease inhibitors, NS5B polymerase inhibitors or NS5A inhibitors) have a higher and pangenotypic antiviral activity, a fair safety profile and a lower pill burden. However, their triple combinations with PR, even if it increases the SVR rate significantly (from 75% to 90%), remains associated with the significant adverse events of the PR combination. This is why it is likely that combinations of these new oral antiviral agents in interferon-free regimens will soon become the standard of care for HCV infection, tailored to individual patients according to the degree of disease progression (fibrosis, cirrhosis, hepatocellular carcinoma), HCV genotypes and subtypes, resistance profiles and prior therapeutic history. This review summarizes most of the recent reported data. However, it is difficult to obtain a complete picture of this new field because: (1) completeness is becoming a challenge given the very rapid development of various drugs and combination; (2) most of the results are preliminary, achieved in a limited number of patients belonging mainly to ‘easy-to-treat’ populations without cirrhosis; (3) unmet needs persist in ‘difficult-to-treat’ patients such as cirrhotics, allograft recipients, human immunodeficiency virus (HIV) co-infected patients or patients with end-stage renal disease; and (4) several agents in the pipeline have had to be discontinued due to safety issues.
Second-wave DAAs
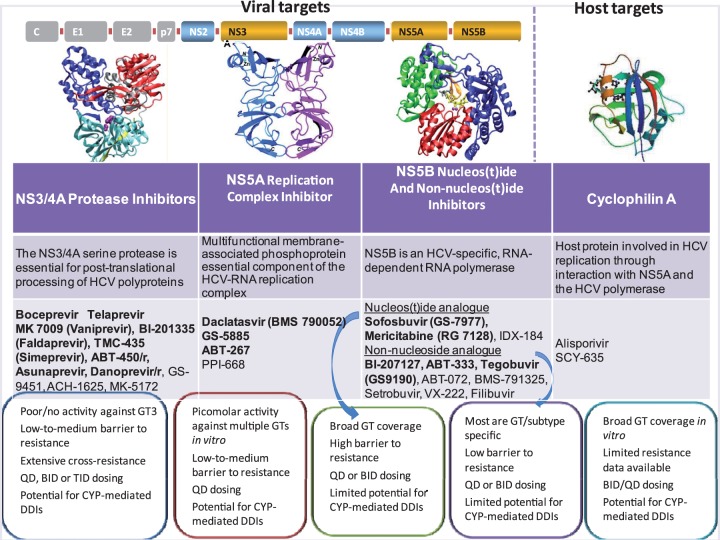
A better understanding of the HCV lifecycle and the characterization of viral enzymes that are potential antiviral targets has led to the development of a number of potential new DAAs targeted against viral proteins (Figure 1) [Buhler and Bartenschlager, 2012; Yang et al. 2011]. These include NS3/NS4A protease inhibitors, nucleos(t)idic or non-nucleos(t)idic NS5B polymerase inhibitors, and NS5A inhibitors. Most of ongoing protocols are summarized in Table 1.
Figure 1.
Potential antiviral targets and new direct-acting antivirals under progress, including NS3/4 protease inhibitors, NS5A nucleos(t)idic or non-nucleos(t)idic polymerase inhibitors and replication complex NS5A inhibitors. Adapted from Moradpour et al. 2007, Pawlotsky et al. 2007, Telluinghuisen et al. 2005, Gish et al. 2001, Coelmont et al. 2010.
BID, twice daily; CYP, cytochrome P450; DDI, drug–drug interaction; GT, genotype; GT3, genotype 3; HCV, hepatitis C virus; QD, once daily; TID, three times a day.
Table 1.
Summary of new direct-acting antivirals (DAAs) in phase III studies for chronic hepatitis C (adapted from last interrogation of ClinTrials.gov on 25 February 2013).
| Company | ClinicalTrials. gov identifier | DAAs | PR* | Status |
|---|---|---|---|---|
| Janssen | NCT01725529 | TMC-435 | PR | Recruiting |
| NCT01727323 | TMC-435 | PR | Not recruiting | |
| NCT01366638 | TMC-435 | PR | Completed | |
| NCT01292239 | TMC-435 | PR | Completed | |
| NCT01290731 | TMC-435 | PR | Completed | |
| NCT01349465 | TMC-435 | PR | Not recruiting | |
| NCT01567735 | TMC-435 | PR | Not recruiting | |
| NCT01281839 | TMC-435 | PR | Not recruiting | |
| NCT01289782 | TMC-435 | PR | Not recruiting | |
| NCT01479868 | TMC-435 | PR | Not recruiting | |
| NCT01290679 | TMC-435 | PR | Not recruiting | |
| NCT01288209 | TMC-435 | PR | Completed | |
| NCT01323244 | TMC-435 | PR | Recruiting | |
| NCT01485991 | TMC-435 | PR | Not recruiting | |
| Abbott | NCT01773070 | ABT-450/r, ABT-333, ABT-267 | R | Not recruiting |
| NCT01716585 | ABT-450/r, ABT-333, ABT-267 | R | Recruiting | |
| NCT01715415 | ABT-450/r, ABT-333, ABT-267 | R | Recruiting | |
| NCT01767116 | ABT-450/r, ABT-333, ABT-267 | R | Recruiting | |
| NCT01704755 | ABT-450/r, ABT-333, ABT-267 | R | Recruiting | |
| Boehringer | NCT01608737 | BI-201335 | PR | Not recruiting |
| NCT01399619 | BI-201335 | PR | Not recruiting | |
| NCT01358864 | BI-201335 | PR | Not recruiting | |
| NCT01330316 | BI-201335 | PR | Recruiting | |
| NCT01297270 | BI-201335 | PR | Not recruiting | |
| NCT01343888 | BI-201335 | PR | Not recruiting | |
| NCT01579474 | BI-201335 | PR | Not recruiting | |
| NCT01728324 | BI-201335, BI-207127 | R | Recruiting | |
| NCT01732796 | BI-201335, BI-207127 | R | Recruiting | |
| BMS | NCT01448044 | BMS-790052 | PR | Not recruiting |
| NCT01616524 | BMS-790052 | PR** | Recruiting | |
| NCT01492426 | BMS-790052 | PR | Not recruiting | |
| NCT01389323 | BMS-790052 | PR | Not recruiting | |
| NCT01471574 | BMS-790052 | PR | Recruiting | |
| NCT01718158 | BMS-790052 | PR** | Not recruiting | |
| NCT01741545 | BMS-790052 | PR** | Not recruiting | |
| NCT01497834 | BMS-790052, BMS-650032 | No | Not recruiting | |
| NCT01718145 | BMS-790052, BMS-650032 | No | Not recruiting | |
| NCT01581203 | BMS-790052, BMS-650032 | No or PR | Recruiting | |
| NCT01573351 | BMS-790052, BMS-650032 | PR | Recruiting | |
| Merck | NCT01370642 | MK-7009 | PR | Not recruiting |
| NCT01405937 | MK-7009 | PR | Not recruiting | |
| NCT01405560 | MK-7009 | PR | Not recruiting | |
| NCT00689390 | SCH 900518 | PR | Recruiting | |
| Gilead | NCT01667731 | GS-7977 | R | Not recruiting |
| NCT01783678 | GS-7977 | R | Recruiting | |
| NCT01542788 | GS-7977 | R | Not recruiting | |
| NCT01604850 | GS-7977 | R | Not recruiting | |
| NCT01641640 | GS-7977 | PR | Not recruiting | |
| NCT01497366 | GS-7977 | R | Not recruiting | |
| NCT01625338 | GS-7977 | R | Recruiting | |
| NCT01682720 | GS-7977 | R | Recruiting | |
| NCT01701401 | GS-7977, GS-5885 | No or R | Recruiting | |
| NCT01768286 | GS-7977, GS-5885 | No or R | Not recruiting |
DAAs alone (No), or associated with pegylated interferon alpha (P) or ribavirin (R) or pegylated interferon alpha + ribavirin (PR).
Comparison also with pegylated interferon lambda.
Second-wave protease inhibitors
After telaprevir and boceprevir, other protease inhibitors including macrocyclic inhibitors, are now in phase II studies: danoprevir (boosted by ritonavir), GS-9256, ABT-450 (boosted by ritonavir), vaniprevir (MK-7009), BI-201335 (faldaprevir), TMC-435 (simeprevir), MK-5172 and asunaprevir.
For vaniprevir, a phase II trial showed that triple therapy with vaniprevir and PR is more efficient than PR in previously treated genotype 1 patients: between 66.7% and 84.6% of SVR (according to the dose of vaniprevir and duration of triple therapy) versus 19% in the control group (p < 0.001) [Manns et al. 2012]. Similar results have been reported for asunaprevir in combination with PR [Bronowicki et al. 211].
Faldaprevir is a peptidomimetic linear protease inhibitor which has a long half-life, as demonstrated by preclinical and human pharmacokinetic studies, allowing once-daily (QD) dosing. In phase Ib studies, faldaprevir combined with PR demonstrated strong antiviral responses and was well tolerated in treatment-naïve and treatment-experienced HCV genotype 1 patients [Sulkowski et al. 2013]. In a phase IIb study of faldaprevir (SILEN-C1), up to 84% of treatment-naïve genotype 1 patients achieved SVR, and the safety and tolerability profile of faldaprevir was found to be favourable. Moreover, up to 87% of patients achieved the criterion of a maintained rapid virologic response (mRVR) (HCV RNA <25 IU/ml at week 4 and undetectable from week 8 to week 20) and qualified for shortened treatment duration with 24 weeks overall treatment. In the SILEN-C3 study, triple therapy with faldaprevir in genotype 1 naïve patients (including cirrhotic patients), during 12 or 24 weeks followed by PR for 12 to 36 weeks according to extended virologic response (eRVR) achievement (defined by undetectability of HCV RNA at week 4 and 12 of therapy) or not, resulted in similar SVR rates between the two groups (65% versus 73%), with a good tolerance [Dieterich et al. 2011]. Faldaprevir is now in phase III (Table 1).
Simeprevir seems especially interesting because of its broader antiviral spectrum since it inhibits the viral replication of genotypes 1, 2, 4, 5, and 6 in vitro, but not genotype 3 [Lenz et al. 2013]. The ASPIRE study assessed triple therapy with simeprevir during 12, 24 or 48 weeks and bitherapy during 48 weeks, in previously treated genotype 1 patients; in the arm simeprevir 150 mg/day SVR was significantly higher compared with the control group (PR 48 weeks) 72.9% versus 22.7% [Lenz et al. 2012]. Interesting results have been reported in experienced patients especially in prior null responders. In relapsers or partial responders, the SVR rate with triple therapy including simeprevir was not clearly different than that reported for triple therapy including telaprevir in the REALIZE trial [Fried et al. 2011]. Finally, the impact of the polymorphism of the protease codon Q80K which is present in 30–40% of genotype 1a viruses has to be better defined, even if the problem may be overcome using high doses of simeprevir.
Replication complex NS5A inhibitors
Daclatasvir (BMS-790052) is a potent and highly selective NS5A replication complex inhibitor with broad genotypic coverage (genotypes 1–5) and a pharmacokinetic profile supportive of QD dosing [Gao et al. 2010]. Daclatasvir is the first NS5A replication complex inhibitor to enter clinical development for the treatment of chronic hepatitis C. Its antiviral efficacy and resistance profile have been reported in phase IIa and IIb studies in combination with PR for 24–48 weeks resulting in around 75% of SVR rate in genotype-1-infected patients (eRVR was 100% in genotype-4-infected patients) [Pol et al. 2012; Hezode et al. 2012b] but poorer results were achieved in experienced patients [Ratziu et al. 2012].
Other NS5A inhibitors have been associated in oral combinations with fascinating results (ABT-264 and GS-5885) and others are in rapid development (MK MK-8742, GS-5885, GSK-2336805, PPI-668).
Polymerase inhibitors
Two different classes of polymerase inhibitors (PolI) are currently developed, nucleosidic (sofosbuvir/GS-7977 or mericitabine) and non-nucleosidic (ABT-333, VX-222, BI-207127, BMS-791325), which may be associated or not with interferon.
The combination of mericitabine with PR resulted in genotype-1-infected patients achieving less than 60% of SVR [Pockros et al. 2012]. In contrast, the combination of sofosbuvir/GS-7977 (400 mg QD) and PR for 12–24 weeks in genotype 1, 4 and 6 infection resulted in an SVR12 (HCV RNA undetectable at week 12 post-treatment) rate higher than 90% with a fair safety profile and a lower pill burden than the PR combination [Kowdley et al. 2013].
All these studies using second-wave DAAs in combination with PR usually resulted in a higher antiviral potency with a better safety profile that those reported with the first-generation HCV protease inhibitors with a lower pill burden (one to two pills per day). Their limitations are mainly the association with interferon and the numerous pegylated interferon alpha or PR associated adverse events and the duration of the treatment (24–48 weeks with a response-guided therapy depending on early viral kinetics). These limitations suggest the need for new trials using new combinations with better antiviral potency and safety/tolerance and without interferon
Oral combinations of DAAs
The combination of several agents with direct antiviral action could, by targeting various steps of viral replication, induce viral suppression while preventing the emergence of viral resistance and allowing eradication of HCV in interferon-free regimens.
Proof of concept
The proof of concept was provided by the INFORM 1 study [Gane et al. 2010], which assessed the combination RG-7128 [mericitabine 1000 mg twice daily (BID)], danoprevir (100 mg boosted by ritonavir 100 mg BID) and ribavirin in 88 genotype 1 naïve noncirrhotic patients for 24 weeks. The main objective was to decrease the viral load by week 14. The median viral load decline ranged from –3.7 to –5.2 log (versus +0.1 log in the placebo control group), with a good tolerance. In patients achieving a rapid viral decline and who were not completed with the PR combination, the SVR12 rate in genotype-1-infected patients was only 41%, 26% in genotype 1a and 71% in genotype 1b with minimal influence of the IL28B genotype [Gane et al. 2010].
In another study, the combination of the protease inhibitor BI 201335 (faldaprevir: 120 mg/day), the polymerase inhibitor BI 207127 (600 mg/day) and ribavirin had a potent antiviral activity against HCV genotype 1 without serious or severe adverse events [Zeuzem et al. 2011b]; a 28-week combination allowed a 68% SVR12 rate (82% in G1b and G1a with IL28B genotype CC but only 32% in non-CC G1a). Interestingly, these encouraging results were similar in cirrhotic patients [Soriano et al. 2012].
These two pioneer studies clearly demonstrated that: (1) oral combination of DAAs may achieve significant SVR rates; (2) subtype 1b is easier to cure than subtype 1a infection with DAAs (around 75% versus 35%) in part related to the resistance profile, though this was previously reported with the PR combination which is assumed to be not associated with viral resistance [Rallón et al. 2012]; (3) the IL28B polymorphism may have an impact in the choice of therapy; and (4) cirrhosis had only a limited negative impact on the SVR rate.
Dual oral combination
The first dual DAA oral combination to be studied was daclatasvir (60 mg QD) plus asunaprevir (600 mg BID) given in 11 patients (nine with HCV genotype 1a) for 24 weeks [Lok et al. 2012]. Only 4/11 patients (36%) achieved SVR12 and SVR24 (HCV RNA undetectable at week 24 post-treatment): 2/9 with genotype 1a and 2/2 with genotype 1b. Viral breakthrough occurred between weeks 3 and 12 in 6/11 patients, all with genotype 1a. Details on the safety and resistance were published recently [Pol, 2013]. The daclatasvir 60 mg QD/asunaprevir combination has been further evaluated in an expansion cohort of 41 patients (20 with asunaprevir 200 mg BID and 21 patients with asunaprevir 200 mg QD) with similar results with a SVR4 (HCV RNA undetectable at week 4 post-treatment) in 13/15 and 10/16 patients, respectively [Pol, 2013].
The combination of daclatasvir (60 mg QD) and asunaprevir (initially 600 mg BID in a sentinel cohort of 10 null responders, subsequently reduced to 200 mg BID) for 24 weeks in an open-label phase IIa study (AI447-017) was given to 43 HCV genotype-1b-infected Japanese patients who were intolerant or ineligible to PR (n = 20) or null responders (n = 21) [Suzuki et al. 2011]. Among null responders, 19/21 (90%) had SVR12 compared with 14/22 (64%) of ineligible or intolerant patients (in whom the plasma trough concentrations were below the median level for both agents, arguing a poor compliance, but rapid virologic response was similar to that achieved in null responders).
The Electron studies combining sofosbuvir/GS-7977 and ribavirin for 12 weeks (in comparison with the sofosbuvir/GS-7977 and PR combination) resulted in a 100% SVR rate in noncirrhotic naïve genotype-2- and genotype-3-infected patients (only 10 patients per arm), but monotherapy by sofosbuvir/GS-7977 for 12 weeks, reduction of the ribavirin dosing (800 mg) or reduction of the treatment duration (8 weeks) reduced the SVR rate (60%, 60% and 67%, respectively) [Gane et al. 2013]. Finally, the 12-week sofosbuvir/GS-7977 and ribavirin combination in 25 experienced patients allowed a 68% SVR rate [Gane et al. 2013].
In the Positron study, 278 genotype 2 and 3 naïve patients were given the 12-week sofosbuvir/GS-7977 (400 mg QD) and ribavirin combination (n = 207) or placebo (n = 71) (16% cirrhotic, 51% genotype 2 and 49% genotype 3); the SVR12 rates were 78% (compared with 0% in the placebo arm) in the overall treated population (but 93% in genotype 2, contrasting with 61% in genotype 3) and 81% in noncirrhotics versus 61% in cirrhotics.
In the Fission study, 499 genotype 2 and 3 naïve patients were given the 12-week sofosbuvir/GS-7977 and ribavirin combination (n = 258) or the PR combination for 24 weeks as the standard of care (n = 243) (20% cirrhotic, 28% genotype 2 and 72% genotype 3). The SVR12 rates were similar with the oral dual new combination and the PR arm (67%), but different in genotype 2 (97 versus 78%) and not different for genotype 3 (56% versus 63%) or cirrhotics (47% versus 38%) (press release Gilead on February 4, 2013; http://www.gilead.com/news/press-releases/2013/2/gilead-announces-sustained-virologic-response-rates-from-two-phase-3-studies-of-sofosbuvir-for-hepatitis-c).
These very recent results provide evidence of the negative impact of genotype 3 on SVR while cirrhosis seems to poorly affect the SVR rate in genotype-2-infected patients. This resulted on 4 February 2013 in an extension to 24 weeks of sofosbuvir/GS-7977 and ribavirin combination in genotype-3-infected patients from the Valence study.
Dual and triple combination
In a first study evaluating the combination of an NS5A inhibitor and a nucleotide NS5B inhibitor, daclatasvir has been studied in combination with the sofosbuvir/GS-7977 in an interferon-free regimen [Sulkowski et al. 2012a]. Treatment-naïve patients with HCV genotype 1, 2 or 3 received daclatasvir (60 mg QD) plus the sofosbuvir (400 mg QD), with or a without a 1 week lead-in of sofosbuvir, with or without ribavirin, over 24 weeks [Kowdley et al. 2013; Gane et al. 2010]. In patients with genotype 1a or 1b, 86–87% (12/14 and 13/15) of patients treated with the dual combination daclatasvir plus GS-7977, and 93% (14/15) of those receiving the triple combination daclatasvir plus GS-7977 plus ribavirin had undetectable HCV RNA at week 24 [end of therapy (EOT)]. All patients achieved SVR4 and SVR12. In patients with HCV genotype 2 or 3, 94–100% (15/16 and 14/14) of patients treated with the dual combination daclatasvir plus GS-7977, and 86% (12/14) of those receiving the triple combination daclatasvir plus GS-7977 plus ribavirin, had undetectable HCV RNA at week 24 (EOT); 88–100% (14/16 and 14/14) of patients treated with the dual combination daclatasvir + GS-7977, and 86% (12/14; two patients were lost to follow up) of those receiving the triple combination daclatasvir plus GS-7977 plus ribavirin achieved SVR4 and SVR 12. Thus, with an all-oral combination of daclatasvir plus GS-7977, SVR12 rates of >95% were achieved independent of HCV genotype. The sofosbuvir/GS-7977 lead-in phase or the addition of ribavirin had no effect on virologic response but the latter increased the frequency of anaemia (which was absent in the ribavirin-free arms).
Quadruple combination and more
The rapid development of ABT-450 boosted by ritonavir in association with the NS5B non-nucleos(t)ide polymerase inhibitor ABT-333 with ribavirin for 12 weeks results in excellent SVR rates in naïve genotype 1 noncirrhotic patients (93 and 95% in 14 and 19 patients) while results were disappointing in 17 prior experienced patients (47% of SVR with a good RVR of 77% indicating a high rate of viral breakthrough) [Poordad et al. 2013].
Preliminary results of another quadruple combination of the protease inhibitor GS-9451, the NS5A inhibitor GS-5885 (30 for 46 patients or 90 mg daily for 94 patients), the non-nucleosidic polymerase inhibitor tegobuvir/GS-9190 and ribavirin for 12–24 weeks suggest a high SVR4 or SVR12 rates from 77% to 100% with higher rates in genotype-1b- than in genotype-1a-infected patients [Sulkowski et al. 2012b].
Finally, fascinating results have been reported in noncirrhotic genotype-1-infected patients who were given a quadruple or quintuple combination ABT-450 boosted by ritonavir, the ABT-267 NS5A inhibitor, in association or not with the NS5B non-nucleos(t)ide polymerase inhibitor ABT-333 and ribavirin for 8–12 weeks [Kowdley et al. 2012]. The SVR12 rate was from 87% to 97% in naïve patients and 93% in experienced null responders, including 100% in subtype 1b patients.
In summary, the data presented in this review show that various DAAs can be combined together to achieve high SVR rates, with a rather good tolerance (even if agents in the pipeline were discontinued given severe adverse events) and a convenient dosing schedule.
After more than 15 years with the combination of PR as the standard treatment for chronic HCV infection, this field is now dramatically changing with the rapid entry of numerous new antivirals into clinical development, including DAAs and agents with nonviral targets (cyclophilin inhibitors, interferon lambda, vaccine therapy). It is likely that combinations of these agents, in interferon-free regimens, will soon become the standard of care for HCV infection, tailored to individual patients according to the degree of disease progression (fibrosis, cirrhosis, hepatocellular carcinoma), HCV genotypes and subtypes, resistance profiles and prior therapeutic history. The hypothesis of a ‘magical combination (i.e. including sofosbuvir)’ is appealing; the results of the triple oral combination by BMS and Abbott, or of the quadruple/quintuple oral combination of the Aviator study (protease inhibitor/r, PolI, NS5A inhibitor and ribavirin), resulting in more than 97% of SVR challenge such a hypothesis. And this remains probably true despite the next combination of sofosbuvir with ribavirin or the NS5A inhibitor GS-5885 in a ‘single-tablet regimen’.
These regimens have a better safety profile and greater antiviral potency compared with the combination of PR and a first-generation protease inhibitor. Future challenges to be addressed, over and above the already increased efficacy, will be to further improve the safety (resistance does not appear to be a major concern) and adherence, and to evaluate the cost-effectiveness of these new oral combinations.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
S.P. has received consulting and lecturing fees from Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, Vertex, Gilead, Roche, Schering-Plough/Merck, Novartis, Abbott, Sanofi, and GlaxoSmithKline, and grants from Bristol-Myers Squibb, Gilead, Roche and Merck/Schering Plough. M.C. has no conflict of interest to declare. P.S. has received fees for workshop participations or meeting invitations from Gilead, Bristol-Myers Squibb, Schering-Plough / MSD, Roche, Janssen and Mayoli-Spindler.
References
- Bacon B., Gordon S., Lawitz E., Marcellin P., Vierling J., Zeuzem S., et al. (2011) Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 364: 1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronowicki J., Pol S., Thuluvath P., Larrey D., Martorell C., Rustgi V., et al. (2011) BMS-650032, an NS3 inhibitor, in combination with peginterferon alpha-2a and ribavirin in treatment-naïve subjects with genotype 1 chronic hepatitis C infection. J Hepatol 54(Suppl. 1): S472–S472 [Google Scholar]
- Buhler S., Bartenschlager R. (2012) New targets for antiviral therapy of chronic hepatitis C. Liver Int 32(Suppl.): 19–16 [DOI] [PubMed] [Google Scholar]
- Burger D., Back D., Buggisch P., Buti M., Craxí A., Foster G., et al. (2013) Clinical management of drug–drug interactions in HCV therapy: challenges and solutions. J Hepatol 58: 792–800 [DOI] [PubMed] [Google Scholar]
- Burioni R., Perotti M., Mancini N., Clementi M. (2008) Perspectives for the utilization of neutralizing human monoclonal antibodies as anti-HCV drugs. J Hepatol 49: 299–300 [DOI] [PubMed] [Google Scholar]
- Cacoub P., Bourlière M., Lübbe J., Dupin N., Buggisch P., Dusheiko G., et al. (2012) Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol 56: 455–463 [DOI] [PubMed] [Google Scholar]
- Coelmont, L., Hanoulle, X., Chatterji, U., Berger, C., Snoeck, J., Bobardt, M. (2010). DEB025 (Alisporivir) inhibits hepatitis C virus replication by preventing a cyclophilin A induced cis-trans isomerisation in domain II of NS5A. PLoS One. 5: e13687. [DOI] [PMC free article] [PubMed]
- Dieterich D., Asselah T., Guyader D., Berg T., Ceausu E., Preotescu L., et al. (2011) Silen-C3: treatment for 12 or 24 weeks with BI201335 combined with Peginterferon alpha-2A and ribavirin (P/R) in treatment-naïve patients with chronic genotype-1 HCV infection. Hepatology 54(Suppl.): 378A–378A21469167 [Google Scholar]
- Donnelly R., Kotenko S. (2010) Interferon-lambda: a new addition to an old family. J. Interferon Cytokine Res 30: 555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the Liver (2011) EASL Clinical Practice Guidelines: management of chronic hepatitis C virus infection. J Hepatol 55: 245–264 [DOI] [PubMed] [Google Scholar]
- Flisiak R., Dumont J., Crabbe R. (2007) Cyclophilin inhibitors in hepatitis C viral infection. Expert Opin Inv Drugs 16: 1345–1354 [DOI] [PubMed] [Google Scholar]
- Foster G., Hezode C., Bronowicki J., Carosi G., Weiland O., Verlinden L., et al. (2011) Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology 141: 881–889 [DOI] [PubMed] [Google Scholar]
- Fried M., Buti M., Dore G., Flisiak R., Ferenci P., Jacobson I., et al. (2011) TMC435 in combination with peginterferon and ribavirin in treatment naïve HCV genotype 1 patients: final analysis of the Pillar phase IIb study. Hepatology 54(Suppl.): 1429A–1429A [Google Scholar]
- Gane E., Roberts S., Stedman C., Angus P., Ritchie B., Elston R., et al. (2010) Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and Danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 376: 1467–1475 [DOI] [PubMed] [Google Scholar]
- Gane E., Stedman C., Hyland R., Ding X., Svarovskaia E., Symonds W., et al. (2013) Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 368: 34–44 [DOI] [PubMed] [Google Scholar]
- Gao M., Nettles R., Belema M., Snyder L., Nguyen V., Fridell R., et al. (2010) Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 465: 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish, R.G., Meanwell, N.A. (2011) The NS5A replication complex inhibitors: difference makers? Clin Liver Dis 15: 627–639. [DOI] [PubMed]
- Hezode, C., Dorival, C., Zoulim, F., Poynard, T., Mathurin, P., Pol, S. et al. (2012a) Safety of telaprevir or boceprevir in combination with peginterferon alpha/ribavirin, in cirrhotic non responders. First results of the French early access program (ANRS C020-CUPIC). J Hepatol 56(Suppl. 2): S4.
- Hezode C., Hirschfield G., Ghesquiere W., Sievert W., Rodriguez-Torres M., Shafran S., et al. (2012b) Daclatasvir, an NS5A replication complex inhibitor, combined with peginterferon alpha-2a and ribavirin in treatment-naïve HCV-genotype 1 or 4 subjects: phase 2b COMMAND-1 SVR12 results [Abstract]. Hepatology 56(Suppl. 4): 553A–554A [Google Scholar]
- Jacobson I., McHutchison J., Dusheiko G., Di Bisceglie A., Reddy K., Bzowej N., et al. (2011) Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 364: 2405–2416 [DOI] [PubMed] [Google Scholar]
- Kowdley, K., Lawitz, E., Crespo, I., Hassanein, T., Davis, M., DeMicco, M. et al. (2013) Sofosbuvir, an NS5 nucleotide polymerase inhibitor, with peginterferon alpha-2a and ribavirin in treatment-naïve patients with hepatitis C genotypes 1, 4, and 6 infection (ATOMIC): an open-label, randomised, multicentre, phase 2 trial. Lancet Infect Dis. DOI: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed]
- Kowdley K., Lawitz E., Poordad F., Cohen D., Nelson D., Zeuzem S., et al. (2012) A 12-week interferon-free treatment regimen with ABT-450/r, ABT-267, ABT-333 and ribavirin achieves SVR12 rates (observed data) of 99% in treatment-naïve patients and 93% in prior null responders with HCV genotype 1 infection. Hepatology 56(Suppl. 4): abstract LB–01 [Google Scholar]
- Lenz, O., Fevery, B., Vijgen, L., Verbeeck, J., Peeters, M., Beumont-Mauviel, M. et al. (2012) TMC435 in patients infected with HCV genotype 1 who have failed previous pegylated interferon/ribavirin treatment: virological analysis of the ASPIRE trial. J Hepatol 56(Suppl. 2): S5.
- Lenz O., Vijgen L., Berke J., Cummings M., Fevery B., Peeters M., et al. (2013) Virologic response and characterisation of HCV genotype 2-6 in patients receiving TMC435 monotherapy (study TMC435-C202). J Hepatol 58: 445–451 [DOI] [PubMed] [Google Scholar]
- Lok A., Gardiner D., Lawitz E., Martorell C., Everson G., Ghalib R., et al. (2012) Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 366: 216–224 [DOI] [PubMed] [Google Scholar]
- Manns M., Gane E., Rodriguez-Torres M., Stoehr A., Yeh C., Marcellin P., et al. (2012) MK-7009 Protocol 007 Study Group. Vaniprevir with pegylated interferon alpha-2a and ribavirin in treatment-naïve patients with chronic hepatitis C: a randomized phase II study. Hepatology 56: 884–893 [DOI] [PubMed] [Google Scholar]
- Moradpour D., Penin F., Rice C. (2007) Replication of hepatitis C virus. Nat Rev Microbiol 5: 453–463 [DOI] [PubMed] [Google Scholar]
- Pawlotsky, J.M., Chevaliez, S., McHutchison, J.G. (2007) The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology 132: 1979–98. [DOI] [PubMed]
- Pockros P., Jensen D., Tsai N., Taylor R., Ramji A., Cooper C., et al. (2012) SVR-12 among G1/4 treatment-naïve patients receiving mericitabine in combination with PEG-IFN a-2A/RBV: interim analysis from the JUMP-C study. J Hepatol 56(Suppl. 2): S477–S478 [Google Scholar]
- Pol S. (2013) Daclatasvir, an efficient inhibitor of the hepatitis C virus replication complex protein NS5A: review of virological data, treatment rationale and clinical trials. Clin Invest 3: 191–208 [Google Scholar]
- Pol S., Ghalib R., Rustgi V., Martorell C., Everson G., Tatum H., et al. (2012) Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis 12: 671–677 [DOI] [PubMed] [Google Scholar]
- Poordad F., Lawitz E., Kowdley K., Cohen D., Podsadecki T., Siggelkow S., et al. (2013) Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med 368: 45–53 [DOI] [PubMed] [Google Scholar]
- Poordad F., McCone J., Jr., Bacon B., Bruno S., Manns M., Sulkowski M., et al. (2011) Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 364: 1195–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallón N., Pineda J., Soriano V., Neukam K., Vispo E., Rivero A., et al. (2012) Differences in virological response to peginterferon-α plus ribavirin in HIV-positive patients coinfected with HCV subtypes 1a or 1b. J Acquired Immune Defic Syndr 60: 117–123 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Gadano A., Pol S., Hézode C., Ramji A., Cheng W., et al. (2012) Triple therapy with daclatasvir (DCV; BMS-790052), peginterferon alpha-2a and ribavirin in HCV-infected prior null and partial responders: 12-week results of phase 2b COMMAND-2 trial. J Hepatol 56(Suppl. 2): S478–S479 [Google Scholar]
- Sarrazin C., Hezode C., Zeuzem S., Pawlotsky J. (2012) Antiviral strategies in hepatitis C virus infection. J. Hepatol 56(Suppl. 1): S88–S100 [DOI] [PubMed] [Google Scholar]
- Soriano V., Gane E., Angus P., Stickel F., Bronowicki J.-P., Roberts S., et al. (2012) The efficacy and safety of the interferon-free combination of BI201335 and BI207127 in genotype 1 HCV patients with cirrhosis. Interim analysis from SOUND-C2. J Hepatol 56(Suppl. 2): S559–S559 [Google Scholar]
- Sulkowski, M., Asselah, T., Lalezari, J., Ferenci, P., Fainboim, H., Legett, B. et al. (2013) Faldaprevir combined with peginterferon alpha-2a and ribavirin in treatment naïve patients with chronic genotype-1 HCV: SILEN-C1 trial. Hepatology. DOI: 10.1002/hep.26276. [DOI] [PubMed]
- Sulkowski M., Gardiner D., Rodriguez-Torres M., Reddy R., Hassanein T., Jacobson I., et al. (2012a) High rate of sustained virologic response with the all-oral Combination of Daclatasvir (NS5A inhibitor) plus Sofosbuvir (nucleotide NS5B inhibitor), with or without ribavirin, in treatment-naïve patients chronically infected with HCV genotype 1, 2, or 3. Hepatology 56(Suppl. 4): abstract LB–02 [Google Scholar]
- Sulkowski M., Rodriguez-Torres M., Lawitz E., Shiffman M., Pol S., Herring R., et al. (2012b) High sustained virologic response rate in treatment-naïve HCV genotype 1a and 1B patients treated for 12 weeks with an interferon-free all oral QUAD regimen: interim results. EASL 2012. J Hepatol 56(Suppl. 2): S560–S560 [Google Scholar]
- Suzuki F., Chayama K., Kawakami Y., Toyota J., Karino Y., Mochida S., et al. (2011) BMS-790052, an NS5A replication complex inhibitor, in combination with peginterferon alpha-2b and ribavirin in Japanese treatment-naïve and nonresponder patients with chronic HCV genotype 1 infection [Abstract]. Hepatology 54(Suppl.): 1441A–1441A [Google Scholar]
- Tellinghuisen, T.L., Marcotrigiano, J., Rice, C.M. (2005) Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature 435: 374–379. [DOI] [PMC free article] [PubMed]
- Yang P., Gao M., Lin K., Liu Q., Villareal V. (2011) Anti-HCV drugs in the pipeline. Curr Opin Virol 1: 607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S., Andreone P., Pol S., Lawitz E., Diago M., Roberts S., et al. (2011a) Telaprevir for retreatment of HCV infection. N Engl J Med 364: 2417–2428 [DOI] [PubMed] [Google Scholar]
- Zeuzem S., Asselah T., Angus P., Zarski J., Larrey D., Müllhaupt B., et al. (2011b) Efficacy of the protease inhibitor BI 201335, the polymerase inhibitor BI 207127 and ribavirin in patients with chronic hepatitis C. Gastroenterology 141: 2047–2055 [DOI] [PubMed] [Google Scholar]