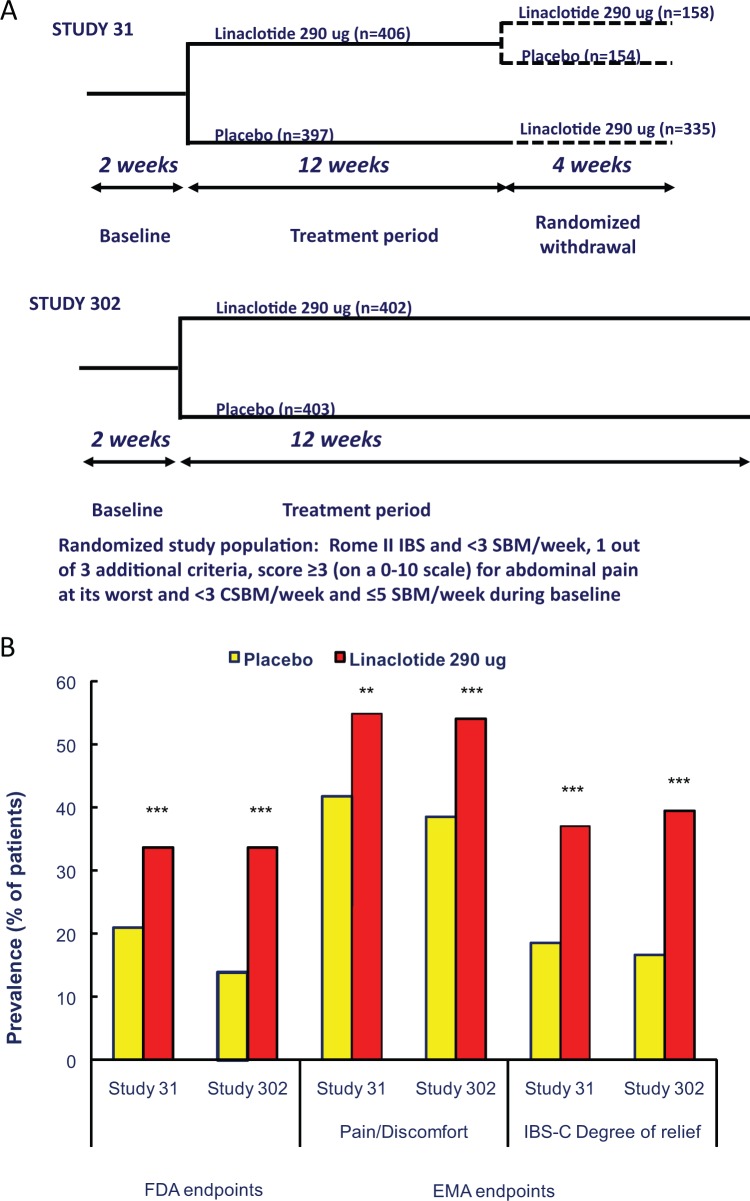
Figure 3.
(A) Schematic outline of the phase III studies with linaclotide in irritable bowel syndrome (IBS) with constipation. SBM: spontaneous bowel movement; CSBM: complete spontaneous bowel movement. (B) Responder rates to placebo and linaclotide 290 µg in studies 31 and 302 in IBS with constipation, according to the Food and Drug administration (FDA) and European Medicines Agency (EMA) guidelines. Left part of the graph: according to FDA guidelines, responders have to be both abdominal pain and bowel movement responders. Abdominal pain responders are defined as patients with a reduction of ≥30% in worst abdominal pain for ≥6/12 first weeks of treatment, and responders for bowel movements are defined as patients with an increase ≥1 CSBM from baseline for ≥6/12 first weeks of treatment. Right part of the graph: according to EMA guidelines, abdominal pain/discomfort responders are defined as patients with a reduction of ≥30% in worst abdominal pain score or mean abdominal discomfort score for that week, with neither of these scores worsening from baseline for that week for ≥6/12 weeks, and IBS degree-of-relief responders were defined as patients whose response to the degree-of-relief of IBS symptoms question was “considerably relieved” or “completely relieved” (i.e. a score of 1 or 2) for ≥6/12 first weeks of treatment. *p < 0.01 compared to placebo; **p < 0.001 compared to placebo; ***p < 0.0001 compared to placebo.