Abstract
We assembled a prospective cohort of 3144 human immunodeficiency virus (HIV) infected children aged <15 years initiating antiretroviral therapy (ART) in Dar es Salaam, Tanzania. The prospective relationships of baseline covariates with growth were examined using linear regression models. ART led to improvement in mean weight-for-age (WAZ), height/length-for-age (HAZ) and weight-for-length or body mass index (WLZ/BMIZ) scores. However, normal HAZ values were not attained over an average follow-up of 17.2 months. After 6 months of ART, underweight (P < 0.001), low CD4 count or percent (P < 0.001), stavudine containing regimens (P = 0.05) and advanced WHO disease stage (P < 0.001) at ART initiation were associated with better WAZ scores. Age >5 years on the other hand was associated with less increase in WAZ score after 6 months of ART (P < 0.001). These findings suggest that although ART improved the growth of the HIV-infected children in Tanzania, adjunct nutritional interventions may be needed to ensure that the growth of these children is optimized to the greatest extent possible.
Introduction
Globally, there were an estimated 34 million people living with human immunodeficiency virus (HIV) in 2010 [1]. In the same period there were 2.7 million new HIV infections and 1.8 million HIV-related deaths [1]. Children account for an estimated 14% of all new HIV infections globally [2], and mother-to-child transmission is the primary mode of acquisition of HIV infection among pediatric patients worldwide [3, 4]. Sub-Saharan Africa (SSA) remains the region most heavily affected by HIV.
Tanzania faces a mature, generalized HIV epidemic. With a total population of around 40 million, the HIV prevalence for adults aged 15–49 in Tanzania stands at 5.7% in 2007/08 representing a slight decline in prevalence from 7% in 2003/04 [5]. By 2007, an estimated 1.4 million adults and children were living with HIV in Tanzania [6]. Among children, approximately 140 000 children under the age of 15 years were living with HIV/AIDS in the same period [6].
Disturbance in growth is a common feature of children with HIV infection [7–10]. Apart from food insecurity, underlying HIV infection pathology and opportunistic infections can result in growth faltering by limiting food intake and increasing resting energy expenditure, among other mechanisms [7]. Poor growth may also be an indicator for treatment failure.
Despite the abundant literature on nutrition and growth in HIV-infected children [11–17], there is limited information on patterns and predictors of growth among pediatric patients receiving antiretroviral therapy (ART) particularly in SSA. Several previous studies were limited by their smaller sample sizes or shorter duration of follow-up [11–14, 17], and only a few studies examined growth response beyond 2 years of ART [15, 16]. Assessment of growth is key to monitoring disease progression [18, 19], and assessing response to treatment [7] particularly in resource-limited settings where laboratory measurements such as viral load and CD4 count may not be easily accessible.
The aim of this study was to describe the growth pattern and examine the predictors of growth among children receiving ART in Dar es Salaam, Tanzania. The Institutional Review Board of the Harvard School of Public Health approved the study.
Methods
Study design, setting and participants
We assembled a prospective cohort of HIV-infected children receiving ART at Management and Development for Health (MDH), a Tanzanian-based organization supporting high quality HIV/AIDS care and treatment services. Following enrollment, patients were evaluated at monthly clinic visits. At each visit, trained nurses measured patient’s weight, height or length, and mid-upper arm circumference (MUAC) using standardized procedures. Patients also had a complete medical history in the preceding month taken and a detailed physical examination carried out by a physician, underwent nutrition and medication adherence counseling, and received ARV refills. Laboratory tests including complete blood count, liver function tests, and CD4+ cells percents and absolute counts were performed routinely every 4 months.
Patients were initiated on antiretroviral treatment according to the most recent National AIDS Control Program (NACP) ART initiation criteria at the time of initiation [20]. Accordingly, children were eligible for ART when presented with a World health Organization (WHO) clinical stage 4 or stage 3, irrespective of the absolute CD4+ cells count or percentage, or WHO stage 1 or stage 2 and severe immunodeficiency (CD4+ count <200 or CD4+ percent <15%). Standard ART in Tanzania was triple therapy consisting of 2 nucleoside reverse transcriptase inhibitors (NRTI) + 1 non-nucleoside reverse transcriptase inhibitors (NNRTI) or 2 NRTI + 1 protease inhibitor (PI) or 3 NRTI [21]. Standard first line regimens thus included zidovudine (AZT) + lamivudine (3TC) + nevirapine (NVP) for children under 3 years old; zidovudine (AZT) + lamivudine (3TC) + efavirenz (EFV) or nevirapine (NVP) for children 3 years old or more; abacavir (ABC) + lamivudine (3TC) + efavirenz (EFV) for children for children 3 years or more or nevirapine (NVP) for children under 3 years; stavudine (d4T) + lamivudine (3TC) + nevirapine (NVP). Standard first line regimens thus included zidovudine (AZT) + lamivudine (3TC) + nevirapine (NVP) for children under 3 years old; zidovudine (AZT) + lamivudine (3TC) + efavirenz (EFV) or nevirapine (NVP) for children 3 years old or more; abacavir (ABC) + lamivudine (3TC) + efavirenz (EFV) for children 3 years or more or nevirapine (NVP) for children under 3 years; stavudine (d4T) + lamivudine (3TC) + nevirapine (NVP). Stavudine was an alternative for AZT in cases of anemia (i.e. hemoglobin <7.5g/dl). To avoid drug interactions, efavirenz was substituted for nevirapine in patients receiving therapy (rifampin) for tuberculosis. ARVs were switched to second line as a result of toxicity or a lack of good clinical response to the regimen. The recommended second line regimens included the combination of didanosine (ddI) + ABC + ritonavir boosted lopinavir (LPV/r) or nelfinavir (NFV).
Statistical analysis
The outcomes for this study were the standardized anthropometric z-scores; weight-for-age (WAZ), height/length-for-age (HAZ) and weight-for-length or body mass index (BMIZ) scores. The z-scores were computed from the measurements of weight, height or length, and child age in months and sex using the 2006 World health Organization (WHO) Child Growth reference standards [21]. A child was considered to be underweight, stunted or wasted when WAZ, HAZ or WLZ/BMIZ score, respectively, was below –2 of the reference population. Using the WHO guidelines, children up to 5 years of age were classified as having low MUAC if they had MUAC <11.5 cm. However, for children older than 5 years, a cut-off of 16.4 cm (10th percentile) was adopted from NHANES (National Health and Nutritional examination Survey) 2003–06 conducted by Centers for Disease Control (CDC) and National Centre for Health Statistics (NCHS) for children older than 5 years.
We first described growth pattern by plotting the mean Z-scores over time. Associations between baseline covariates and the change in Z-scores after 6 months of ART, point at which the growth patterns stabilize, were then examined using linear regression with the change as a continuous variable. Variables were included in the multivariate models if they were significantly associated with the outcomes in univariate analyses (P < 0.20) or if they were believed to be mechanistically relevant. The missing indicators method was used for covariates with more than 1% of missing data. Adjusted differences in WAZ, HAZ, WLZ/BMIZ and their corresponding 95% confidence intervals (CI) were constructed. The criterion for significance for all analyses was a P-value significant at level of α = 0.05. All P-values were two tailed.
All statistical analyses were performed with the statistical software package SAS (version 9.2, SAS Institute Inc., Cary, NC).
Results
A total of 3180 children <15 years were initiated on ART between October 2004 and December 2010. Thirty six out of these children had invalid or missing information on age and were excluded from the analyses. The remaining sample of 3144 children contributed a median of 17.2 months of follow-up (interquartile range [IQR], 4.6–31.4 months). At ART initiation, 51% were 5 years old or younger, and 50% were girls. The prevalence of underweight was 40% while 30% of the children were wasted, 52% had stunting and 39% had low MUAC. All the children were initiated on the recommended first line ARVs regimens. The rest of the general characteristics of the study population are shown in Table 1.
Table 1.
Demographic and clinical characteristics of 3144 HIV-infected children at the time of ART initiation in Dar es Salaam, Tanzania (2004–10)
| Characteristic | No. of patients (%a) |
|---|---|
| Age (years) | |
| 0–2 | 927 (29) |
| >2–5 | 687 (22) |
| >5 | 1530 (49) |
| Sex | |
| Male | 1562 (50) |
| Female | 1582 (50) |
| Year of ART initiation | |
| 2004–05 | 204 (6) |
| 2006 | 222 (7) |
| 2007 | 617 (20) |
| 2008 | 841 (27) |
| 2009 | 736 (23) |
| 2010 | 524 (17) |
| Underweight (WAZ < –2 SD) | |
| Yes | 1270 (40) |
| No | 1147 (36) |
| Missing | 727 (23) |
| Wasting (WLZ < –2 SD if ≤2 years; BMIZ < –2 SD if >2 years) | |
| Yes | 946 (30) |
| No | 1968 (63) |
| Missing | 230 (7) |
| Stunting (HAZ < –2 SD) | |
| Yes | 1639 (52) |
| No | 1298 (41) |
| Missing | 207 (7) |
| Low MUAC (11.5 cm if ≤ 5 years; 16.4 if >5 years)b | |
| Yes | 1222 (39) |
| No | 1880 (60) |
| Missing | 42 (1) |
| Hemoglobin level (g/dl) | |
| >11 | 403 (13) |
| 8.5–11 | 1281 (41) |
| <8.5 | 502 (16) |
| Missing | 958 (30) |
| Severe immune suppression (CD4 + T-cells <15% if ≤5 years; <200 cells/μl if >5 years)c | |
| Yes | 1035 (33) |
| No | 1162 (37) |
| Missing | 947 (30) |
| WHO disease stage | |
| I | 266 (8) |
| II | 479 (15) |
| III | 1538 (49) |
| IV | 224 (7) |
| Missing | 637 (20) |
| Past history of TB | |
| Yes | 612 (19) |
| No | 2472 (79) |
| Missing | 60 (2) |
| Opportunistic infectionsd | |
| Yes | 360 (11) |
| No | 2772 (88) |
| Missing | 12 (0.4) |
| Use of cotrimoxazole | |
| Yes | 1580 (50) |
| No | 1390 (44) |
| Missing | 174 (6) |
| ARV regimen | |
| Contains stavudine | 761 (24) |
| No stavudine | 1287 (41) |
| Missing | 1096 (35) |
| ARV regimen | |
| Contains efavirenz | 356 (11) |
| No efavirenz | 1692 (54) |
| Missing | 1096 (35) |
| District of residence | |
| Ilala | 1372 (44) |
| Kinondoni | 888 (28) |
| Temeke | 824 (26) |
| Missing | 60 (2) |
aPercents may not add up to 100 because of rounding.
bThere are no established MUAC cut-offs for children >5 years. The 16.4 cm reference (10th percentile) was adopted from NHANES 2003–06 conducted by CDC, NCHS (McDowell, MA).
cAdopted from CDC. Guidelines for Preventing Opportunistic Infections Among HIV-Infected Persons – 2002. MMWR 14 June 2002 (CDC).
dPatient currently having at least one or more of the following: herpes zoster, fungal infection, oral candidiasis, oral hairy leukoplakia, recurrent severe bacterial infection, pneumocystis jirovecii pneumonia, toxoplasmosis, cryptosporidiosis with diarrhea, cryptococcal meningitis, extrapulmonary TB, lymphoma, kaposis sarcoma and HIV encephalopathy.
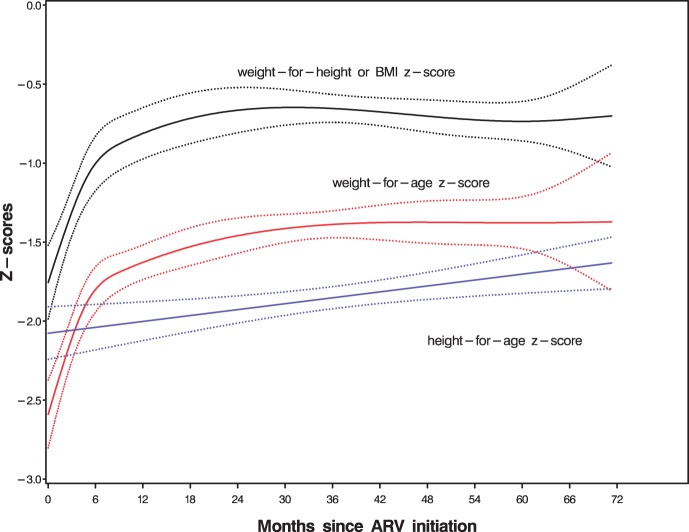
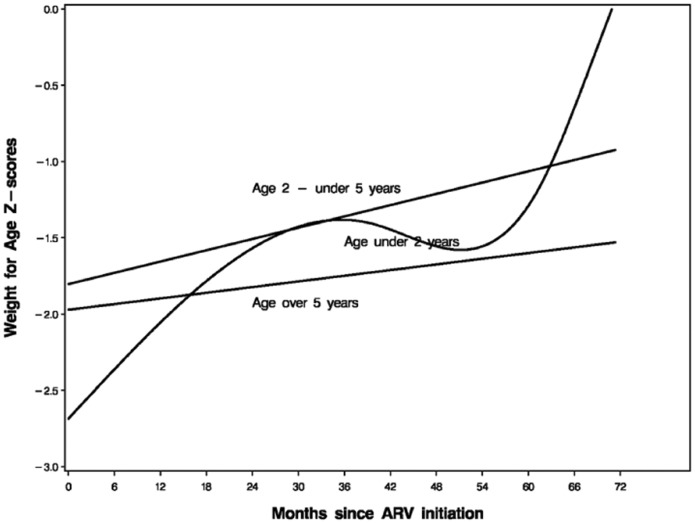
The mean WAZ, HAZ and BMIZ Z-scores and their 95% CI during the period of follow-up are shown in Fig. 1. Administration of ART on average led to an increase in mean Z-scores. However, there was a plateau in growth after 6 months of ART. The age-stratified mean WAZ Z-scores pattern over time are shown in Fig. 2. Administration of ART on average led to an increase in mean WAZ scores with greater effect among children below 2 years of age (P < 0.001) even though they started worse off. However, there was less increase in WAZ scores for children over 5 years of age. Similarly, ART led to improvement in mean HAZ and WLZ/BMIZ scores with greater effect on younger children (P < 0.001). However, only WAZ and WLZ/BMIZ scores reached or approached normal values. Normal HAZ scores on the other hand, were not attained over an average follow-up of 17.2 months.
Fig. 1.
Mean WAZ, HAZ and BMIZ Z-scores and 95% CI with time.
Fig. 2.
Age-stratified mean WAZ Z-score pattern over time.
Table 2 shows the factors associated with change in mean WAZ scores after 6 months of ART initiation. After 6 months of ART, underweight (mean change, 0.78 (95% CI: 0.67, 0.88; P < 0.001), low CD4 count or percent (mean change, 0.29 (95% CI: 0.18, 0.41; P < 0.001), stavudine containing regimens (mean change, 0.12 (95% CI: 0.001, 0.24; P = 0.05), anemia (mean change, 0.17 (95% CI: 0.02, 0.31; P = 0.02) and advanced WHO disease (P < 0.001) at ART initiation were associated with better WAZ scores change. Age>5 years on the other hand was associated with poorer WAZ score change after 6 months of ART (mean change, –0.30 (95% CI: –0.43, –0.17; <0.001).
Table 2.
Predictors of WAZ score change after 6 months of ARTa
| Predictor | Number | Univariate | P | Number | Multivariateb | P |
|---|---|---|---|---|---|---|
| Disease stage | 1694/3144 | <0.001 | 1694/3144 | <0.001 | ||
| I | Reference | Reference | ||||
| II | 0.07 (–0.09, 0.23) | 0.13 (–0.04, 0.30) | ||||
| III | 0.30 (0.17, 0.42) | 0.22 (0.08, 0.37) | ||||
| IV | 0.69 (0.39, 1.00) | 0.45 (0.16, 0.74) | ||||
| ARV regimen | 1694/3144 | 0.02 | 1694/3144 | 0.05 | ||
| No stavudine | Reference | Reference | ||||
| Contains stavudine | 0.15 (0.02, 0.28) | 0.12 (0.001, 0.24) | ||||
| ARV regimen | 1694/3144 | 0.78 | 1694/3144 | 0.89 | ||
| No efavirenz | Reference | Reference | ||||
| Contains efavirenz | –0.02 (–0.19, 0.14) | 0.01 (–0.15, 0.17) | ||||
| Anemia, g/dlc | 1694/3144 | 0.001 | 1694/3144 | 0.02 | ||
| No | Reference | Reference | ||||
| Yes | 0.27 (0.11, 0.44) | 0.17 (0.02, 0.31) | ||||
| Age, years | 1694/3144 | 0.001 | 1694/3144 | <0.001 | ||
| 0–2 | Reference | Reference | ||||
| >2–5 | –0.01 (–0.15, 0.14) | –0.07 (–0.21, 0.06) | ||||
| >5 | –0.22 (–0.36, –0.09) | –0.30 (–0.43, –0.17) | ||||
| Use of cotrimoxazole | 1694/3144 | 0.77 | 1694/3144 | 0.37 | ||
| No | Reference | Reference | ||||
| Yes | 0.02 (–0.09, 0.12) | –0.05 (–0.15, 0.06) | ||||
| Opportunistic infectionsd | 1694/3144 | 0.07 | 1694/3144 | 0.41 | ||
| No | Reference | Reference | ||||
| Yes | 0.16 (–0.01, 0.33) | 0.07 (–0.09, 0.23) | ||||
| Pulmonary TB | 1694/3144 | 0.21 | 1694/3144 | 0.70 | ||
| No | Reference | Reference | ||||
| Yes | 0.08 (–0.04, 0.20) | 0.02 (–0.10, 0.15) | ||||
| Severe immune suppression (CD4+ T-cells < 15% if ≤5 years; <200 cells/μl if >5 years)e | 1694/3144 | <0.001 | 1694/3144 | <0.001 | ||
| No | Reference | Reference | ||||
| Yes | 0.37 (0.25, 0.50) | 0.29 (0.18, 0.41) | ||||
| Underweight at ART initiation (WAZ < –2 SD) | 1694/3144 | <0.001 | 1694/3144 | <0.001 | ||
| No | Reference | Reference | ||||
| Yes | 0.82 (0.73, 0.92) | 0.78 (0.67, 0.88) |
aLinear regression models.
bAdjusted for factors indicated in the table plus gender, district of residence and calendar year.
cHemoglobin: <11 g/dl (age < 5 years); <11.5 g/dl (age 5–11 years); <12 g/dl (age 12–14 years).
dPatient having at least one or more of the following: herpes zoster, fungal infection, oral candidiasis, oral hairy leukoplakia, recurrent severe bacterial infection, pneumocystis jirovecii pneumonia, toxoplasmosis, cryptosporidiosis with diarrhea, cryptococcal meningitis, extrapulmonary TB, lymphoma, kaposis sarcoma, HIV encephalopathy.
eAdopted from CDC. Guidelines for Preventing Opportunistic Infections Among HIV-Infected Persons – 2002. MMWR 14 June 2002 (CDC).
Similarly, wasting, low CD4 count or percent, stavudine containing regimens and advanced WHO disease stage at ART initiation were associated with better WLZ/BMIZ Z-scores while age >5 years and cotrimoxazole use (P = 0.02) were associated with less increase in Z-scores. Stunting and age >2 years at ART initiation were associated with better HAZ scores after 6 months of ART (Table 3). Similarly, wasting, low CD4 count or percent, stavudine containing regimens, and advanced WHO disease stage at ART initiation were associated with better WLZ/BMIZ Z scores while age >5 years and cotrimoxazole use (P = 0.02) were associated with less increase in Z-scores (Table 4).
Table 3.
Predictors of HAZ score change after 6 months of ARTa
| Predictor | Number | Univariate | P | Number | Multivariateb | P |
|---|---|---|---|---|---|---|
| Disease stage | 2133/3144 | <0.001 | 2133/3144 | 0.05 | ||
| I | Reference | Reference | ||||
| II | 0.17 (0.04, 0.29) | 0.11 (–0.03, 0.26) | ||||
| III | 0.13 (0.03, 0.23) | 0.04 (–0.09, 0.16) | ||||
| IV | 0.31 (0.15, 0.48) | 0.16 (–0.03, 0.34) | ||||
| ARV regimen | 2133/3144 | 0.84 | 2133/3144 | 0.25 | ||
| No stavudine | Reference | Reference | ||||
| Contains stavudine | 0.01 (–0.10, 0.08) | –0.06 (–0.15, 0.04) | ||||
| ARV regimen | 2133/3144 | 0.39 | 2133/3144 | 0.11 | ||
| No efavirenz | Reference | Reference | ||||
| Contains efavirenz | –0.05 (–0.15, 0.06) | –0.09 (–0.20, 0.02) | ||||
| Anemia, g/dlc | 2133/3144 | 0.37 | 2133/3144 | 0.23 | ||
| No | Reference | Reference | ||||
| Yes | –0.06 (–0.19, 0.07) | –0.08 (–0.21, 0.05) | ||||
| Age, years | 2133/3144 | 0.02 | 2133/3144 | 0.01 | ||
| 0–2 | Reference | Reference | ||||
| >2–5 | 0.46 (0.33, 0.59) | 0.38 (0.25, 0.51) | ||||
| >5 | 0.18 (0.07, 0.29) | 0.18 (0.06, 0.30) | ||||
| Use of cotrimoxazole | 2133/3144 | 0.55 | 2133/3144 | 0.54 | ||
| No | Reference | Reference | ||||
| Yes | –0.02 (–0.11, 0.06) | –0.03 (–0.11, 0.06) | ||||
| Opportunistic infectionsd | 2133/3144 | 0.77 | 2133/3144 | 0.85 | ||
| No | Reference | Reference | ||||
| Yes | –0.02 (–0.14, 0.10) | –0.01 (–0.14, 0.11) | ||||
| Pulmonary TB | 2133/3144 | 0.12 | 2133/3144 | 0.58 | ||
| No | Reference | Reference | ||||
| Yes | 0.07 (–0.02, 0.16) | 0.03 (–0.07, 0.13) | ||||
| Severe immune suppression (CD4+ T-cells < 15% if ≤5 years; <200 cells/μl if >5 years)e | 2133/3144 | 0.13 | 2133/3144 | 0.52 | ||
| No | Reference | Reference | ||||
| Yes | 0.07 (–0.02, 0.16) | –0.03 (–0.11, 0.06) | ||||
| Stunting at ART initiation (HAZ < –2 SD) | 2133/3144 | <0.001 | 2133/3144 | <0.001 | ||
| No | Reference | Reference | ||||
| Yes | 0.44 (0.36, 0.52) | 0.42 (0.34, 0.50) |
aLinear regression models.
bAdjusted for factors indicated in the table plus gender, district of residence and calendar year.
cHemoglobin: < 11 g/dl (age < 5 years); <11.5 g/dl (age 5–11 years); <12 g/dl (age 12–14 years).
dPatient having at least one or more of the following: herpes zoster, fungal infection, oral candidiasis, oral hairy leukoplakia, recurrent severe bacterial infection, pneumocystis jirovecii pneumonia, toxoplasmosis, cryptosporidiosis with diarrhea, cryptococcal meningitis, extrapulmonary TB, lymphoma, kaposis sarcoma and HIV encephalopathy.
eAdopted from CDC. Guidelines for Preventing Opportunistic Infections Among HIV-Infected Persons – 2002. MMWR 14 June 2002 (CDC).
Table 4.
Predictors of WLZ or BMIZ score change after 6 months of ARTa
| Predictor | Number | Univariate | P | Number | Multivariateb | P |
|---|---|---|---|---|---|---|
| Disease stage | 2084/3144 | <0.001 | 2084/3144 | <0.001 | ||
| I | Reference | Reference | ||||
| II | 0.004 (–0.20, 0.21) | 0.13 (–0.08, 0.34) | ||||
| III | 0.27 (0.11, 0.43) | 0.27 (0.09, 0.46) | ||||
| IV | 0.91 (0.57, 1.25) | 0.49 (0.16, 0.81) | ||||
| ARV regimen | 2084/3144 | 0.01 | 2084/3144 | 0.001 | ||
| No stavudine | Reference | Reference | ||||
| Contains stavudine | 0.22 (0.06, 0.37) | 0.25 (0.11, 0.40) | ||||
| ARV regimen | 2084/3144 | 0.24 | 2084/3144 | 0.52 | ||
| No efavirenz | Reference | Reference | ||||
| Contains efavirenz | 0.12 (–0.08, 0.32) | 0.06 (–0.13, 0.25) | ||||
| Anemia, g/dlc | 2084/3144 | 0.001 | 2084/3144 | 0.001 | ||
| No | Reference | Reference | ||||
| Yes | 0.40 (0.16, 0.64) | 0.37 (0.14, 0.59) | ||||
| Age, years | 2084/3144 | <0.001 | 2084/3144 | <0.001 | ||
| 0–2 | Reference | Reference | ||||
| >2–5 | –0.26 (–0.47, –0.04) | –0.06 (–0.26, 0.14) | ||||
| >5 | –0.31 (–0.49, –0.14) | –0.33 (–0.50, –0.16) | ||||
| Use of cotrimoxazole | 2084/3144 | 0.29 | 2084/3144 | 0.02 | ||
| No | Reference | Reference | ||||
| Yes | –0.07 (–0.21, 0.06) | –0.15 (–0.28, –0.02) | ||||
| Opportunistic infectionsd | 2084/3144 | 0.05 | 2084/3144 | 0.79 | ||
| No | Reference | Reference | ||||
| Yes | 0.21 (0, 0.42) | –0.03 (–0.17, 0.22) | ||||
| Pulmonary TB | 2084/3144 | 0.62 | 2084/3144 | 0.34 | ||
| No | Reference | Reference | ||||
| Yes | 0.04 (–0.12, 0.21) | –0.08 (–0.24, 0.08) | ||||
| Severe immune suppression (CD4+ T-cells < 15% if ≤5 years; <200 cells/μl if >5 years)e | 2084/3144 | <0.001 | 2084/3144 | 0.004 | ||
| No | Reference | Reference | ||||
| Yes | 0.36 (0.21, 0.50) | 0.20 (0.07, 0.34) | ||||
| Wasting at ART initiation (WLZ or BMIZ < –2 SD) | 2084/3144 | <0.001 | 2084/3144 | <0.001 | ||
| No | Reference | Reference | ||||
| Yes | 1.52 (1.38, 1.66) | 1.49 (1.34, 1.63) |
aLinear regression models.
bAdjusted for factors indicated in the table plus gender, district of residence and calendar year.
cHemoglobin: <11 g/dl (age < 5 years); < 11.5 g/dl (age 5–11 years); <12 g/dl (age 12–14 years).
dPatient having at least one or more of the following: herpes zoster, fungal infection, oral candidiasis, oral hairy leukoplakia, recurrent severe bacterial infection, pneumocystis jirovecii pneumonia, toxoplasmosis, cryptosporidiosis with diarrhea, cryptococcal meningitis, extrapulmonary TB, lymphoma, kaposis sarcoma and HIV encephalopathy.
eAdopted from CDC. Guidelines for Preventing Opportunistic Infections Among HIV-Infected Persons – 2002. MMWR 14 June 2002 (CDC).
Discussion
The results of our study indicate that child anthropometric Z-score profiles improved after ART initiation, however, only WAZ, and WLZ or BMIZ scores reached or approached normal values. HAZ scores did not reach the normal values even after 6 years of follow-up. The growth effect was on average greater in younger than older children even though the younger children always started worse off.
Undernutrition at ART initiation as defined by underweight, stunting and wasting was consistently associated with better Z-scores change after 6 months of ART. Our results concur and extend data from previous studies done in Africa and beyond [15, 22–24]. Nachman et al. [22] in their study of children up to 17 years of age and who had received ART for at least 16 weeks reported that children with weight below the 50th percentile for age and gender exhibited relatively more improvement than did those with weights above the 50th percentile. In a study from Zambia, children who were underweight at ART initiation experienced a greater increase in WAZ in the first 6 months of ART [23]. Similarly, Gsponer et al. [15] found that lower baseline WAZ, HAZ and WLZ were the most important determinants of faster catch-up growth on ART. In our study, normal HAZ score were not achieved despite long duration of treatment. Similar findings have been reported in other studies [15, 24]. However, our results contrast the findings from a study in the USA where normal HAZ were reached after 2 years of ART, though the baseline Z-scores were much higher in these children [22].
Advanced HIV disease stage was associated with better WAZ scores change in our study. Similar to our findings, a study from Southern Africa also reported that advanced stage of the disease was predictive of faster catch-up growth in WAZ [15]. In another study, Diniz et al. [25] reported a greater weight and height catch-up after starting HAART in patients with clinically advanced disease at baseline.
We found that a low CD4+ T-cells count or percent were significantly associated with better WAZ scores change after 6 months of ART in this population. A study comparing factors associated with initial growth, CD4 and viral load responses to ART in HIV-infected children in Kampala, Uganda and the UK/Ireland found that children with low pre-ART CD4% had poorer immune responses in Kampala compared with those in the UK/Ireland [26].
Anemia at ART initiation was associated with better WAZ scores change after 6 months of ART in this study. Similarly, in a study by McGrath et al. [27], low baseline hemoglobin (<9 g/dl) was associated with more rapid improvements in WLZ. In the latter study, however, low baseline hemoglobin was not associated WAZ changes [27].
Older children (age >5 years) showed poorer WAZ score change after 6 months of ART compared with younger children in our study. A study by Nachman et al. [22] reported a similar trend. In their trial, subjects <2 years of age though entered the study with the worst height growth; they exhibited faster improvement during the 48 weeks than did older children [22]. Younger age at ART initiation was a predictor of greater weight and height gain in a study in Brazil [25]. Similar findings have been described for weight gain [14, 23, 27, 28]. Better growth outcomes among younger children could be related to the duration of infection. Younger children may have less severe gastrointestinal impairment due to a shorter duration of HIV infection therefore better nutrients absorption after viral suppression by ART [27, 29]. The longer duration of HIV infection in older children may further require more time to reverse the damage implying a more prolonged metabolic cost [27]. Patients who received stavudine containing regimens at ART initiation had better WAZ scores change after 6 months of ART. This effect of stavudine could be mediated through body fat redistribution, a side effect of stavudine and a few other antiretroviral drugs [30, 31]. There was no association between WAZ score change in after 6 months of ART and receiving efavirenz containing regimens at ART initiation. Recent studies have demonstrated that efavirenz may contribute as a side effect, to adipose tissue alterations in patients receiving ART as well [31–33].
There was no association between cotrimoxazole use and WAZ score change after 6 months of ART in this study. A study in Zambia found that, children taking cotrimoxazole had significantly slower decreases in WAZ and HAZ than did children taking placebo [34]. However, the children in the latter study were not on ART. Cotrimoxazole is a broadspectrum antibiotic that has been found to reduce morbidity and mortality among HIV-infected children when taken daily as prophylaxis [35, 36].
Patients with opportunistic infections may have limited food intake and increased energy expenditure leading to poor growth. However, in this study, opportunistic infections and pulmonary TB at the time of ART initiation were not associated with growth outcomes after 6 months of ART in this population.
The current study has a couple of limitations; first, the timing of HIV infection was not known, and residual confounding by this and other unrecorded measures of disease stage was difficult to exclude; second, duration on ART was not long enough and future studies should assess this in a longer duration on ART. Furthermore, deaths and lost to follow-up could be potential sources of bias in our findings; the estimated cumulative mortality rates were 4.8%, 7.3% and 8.5% at 3, 12 and 60 months, respectively, while the cumulative lost to follow-up rates at 3, 12 and 60 months after ART initiation were, respectively, 10.6%, 21.1% and 58.2%. However, there was no difference in the results comparing models weighted for deaths and lost to follow-up using inverse probability weights and unweighted models. The major strength of our study is that its population consisted of a wide age range, belonged to diverse socioeconomic levels. We suggest that our results are generalizable to the population of HIV-infected children receiving ART in Tanzania as well as in other resource-limited countries.
In conclusion, ART led to improvement in mean WAZ, HAZ and WLZ or BMIZ scores. However, there was no catch-up growth on HAZ for all the children over the duration of follow-up. These findings suggest that although ART improved the growth of the HIV-infected children in Tanzania, nutritional interventions and other therapeutic strategies may be needed to ensure that the growth of these children is optimized to the greatest extent possible.
Funding
Fogarty AIDS International Training and Research Program (AITRP) of the National Institutes of Health/Fogarty International Center [grant number 5D43 TW000004 to R.S.M.]. C.D. was partly supported by National Institute of Child Health and Human Development (NICHD) [grant number NICHD K24 HD058795].
Acknowledgements
We thank the nursing and technical staff in Dar es Salaam, Tanzania; and the various scientists at Harvard School of Public Health and Muhimbili University of Health and Allied Sciences for their collaboration and assistance in this study.
References
- 1. WHO/UNAIDS/UNICEF. 2011 Progress report on global HIV/AIDS response. http://www.who.int/hiv/pub/progress_report2011/en/index.html (20 December 2013, date last accessed)
- 2.Kline MW. Perspectives on the pediatric HIV/AIDS pandemic: catalyzing access of children to care and treatment. Pediatrics. 2006;117:1388–93. doi: 10.1542/peds.2005-1348. [DOI] [PubMed] [Google Scholar]
- 3.Diack MBaye A, Signate Sy H, Diagne Gueye NR, et al. Epidemiological and clinical aspects of paediatric HIV infections in Albert-Royer Paediatric Hospital (Dakar, Senegal) Arch Pediatr. 2005;12:404–9. doi: 10.1016/j.arcped.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Bulterys M, Lepage P. Mother-to-child transmission of HIV. Curr Opin Pediatr. 1998;10:143–50. doi: 10.1097/00008480-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 5. TACAIDS, ZAC, NBS, OCGS and Macro International Inc. Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and Macro International Inc. 2008. Tanzania HIV/AIDS and Malaria Indicator Survey 2007-08. 2008. http://www.nbs.go.tz/THIS/THIS2007-08/THIS2007/THMIS%202007%20COMPLETE%20REPORT.pdf (11 November 2008, date last accessed)
- 6. UNAIDS/WHO. Epidemiological Fact Sheets on HIV and AIDS, 2008 Update. 2009:4–21.
- 7.Verweel G, van Rossum AM, Hartwig NG, et al. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics. 2002;109:E25. doi: 10.1542/peds.109.2.e25. [DOI] [PubMed] [Google Scholar]
- 8.Newell ML, Borja MC, Peckham C, et al. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111:e52–60. doi: 10.1542/peds.111.1.e52. [DOI] [PubMed] [Google Scholar]
- 9.Moye J, Jr, Rich KC, Kalish LA, et al. Natural history of somatic growth in infants born to women infected by human immunodeficiency virus. Women and Infants Transmission Study Group. J. Pediatr. 1996;128:58–69. doi: 10.1016/s0022-3476(96)70428-6. [DOI] [PubMed] [Google Scholar]
- 10.Fausto MA, Carneiro M, Antunes CM, et al. Longitudinal growth of infants born to HIV-1-infected mothers in Belo Horizonte, Brazil. Public Health Nutr. 2009;12:783–8. doi: 10.1017/S136898000800267X. [DOI] [PubMed] [Google Scholar]
- 11.Ble C, Floridia M, Muhale C, et al. Efficacy of highly active antiretroviral therapy in HIV-infected, institutionalized orphaned children in Tanzania. Acta Paediatr. 2007;96:1090–4. doi: 10.1111/j.1651-2227.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 12.Villamor E, Fataki MR, Bosch RJ, et al. Human immunodeficiency virus infection, diarrheal disease and sociodemographic predictors of child growth. Acta Paediatr. 2004;93:372–9. [PubMed] [Google Scholar]
- 13.Taye B, Shiferaw S, Enquselassie F. The impact of malnutrition in survival of HIV infected children after initiation of antiretroviral treatment (ART) Ethiop Med J. 2010;48:1–10. [PubMed] [Google Scholar]
- 14.Fassinou P, Elenga N, Rouet F, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Cote d’Ivoire. AIDS. 2004;18:1905–13. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
- 15.Gsponer T, Weigel R, Davies MA, et al. Variability of growth in children starting antiretroviral treatment in Southern Africa. Pediatrics. 2012;130:e966–77. doi: 10.1542/peds.2011-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 17.Eley B, Davies MA, Apolles P, et al. Antiretroviral treatment for children. S Afr Med J. 2006;96:988–93. [PubMed] [Google Scholar]
- 18.Bobat R, Coovadia H, Moodley D, et al. Growth in early childhood in a cohort of children born to HIV-1-infected women from Durban, South Africa. Ann Trop Paediatr. 2001;21:203–10. doi: 10.1080/02724930120077772. [DOI] [PubMed] [Google Scholar]
- 19.Fontana M, Zuin G, Plebani A, et al. Body composition in HIV-infected children: relations with disease progression and survival. Am J Clin Nutr. 1999;69:1282–6. doi: 10.1093/ajcn/69.6.1282. [DOI] [PubMed] [Google Scholar]
- 20.Ministry of Health and Social Welfare, The United Republic of Tanzania. National Guidelines for the Management of HIV and AIDS. Nationa AIDS Control Programme (NACP). 3rd edn. 2008 [Google Scholar]
- 21.World Health Organization. (2006) The WHO Child Growth Standards. http://www.who.int/childgrowth/standards/en/ (20 December 2013, date last accessed) [Google Scholar]
- 22.Nachman SA, Lindsey JC, Moye J, et al. Growth of human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2005;24:352–7. doi: 10.1097/01.inf.0000157095.75081.43. [DOI] [PubMed] [Google Scholar]
- 23.Sutcliffe CG, van Dijk JH, Munsanje B, et al. Weight and height z-scores improve after initiating ART among HIV-infected children in rural Zambia: a cohort study. BMC Infect Dis. 2011;11:54. doi: 10.1186/1471-2334-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillen S, Ramos JT, Resino R, et al. Impact on weight and height with the use of HAART in HIV-infected children. Pediatr Infect Dis J. 2007;26:334–8. doi: 10.1097/01.inf.0000257427.19764.ff. [DOI] [PubMed] [Google Scholar]
- 25.Diniz LM, Maia MM, Camargos LS, et al. Impact of HAART on growth and hospitalization rates among HIV-infected children. J Pediatr (Rio J) 2011;87:131–7. doi: 10.2223/JPED.2064. [DOI] [PubMed] [Google Scholar]
- 26.Kekitiinwa A, Lee KJ, Walker AS, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49:384–92. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 27.McGrath CJ, Chung MH, Richardson BA, et al. Younger age at HAART initiation is associated with more rapid growth reconstitution. AIDS. 2011;25:345–55. doi: 10.1097/QAD.0b013e32834171db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eley B, Nuttall J, Davies MA, et al. Initial experience of a public sector antiretroviral treatment programme for HIV-infected children and their infected parents. S Afr Med J. 2004;94:643–6. [PubMed] [Google Scholar]
- 29.Miller TL, Agostoni C, Duggan C, et al. Gastrointestinal and nutritional complications of human immunodeficiency virus infection. J Pediatr Gastroenterol Nutr. 2008;47:247–53. doi: 10.1097/MPG.0b013e318181b254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres RA, Cadman JA. Potential of recombinant human growth hormone in HIV associated adipose redistribution syndrome. BioDrugs. 2000;14:83–91. doi: 10.2165/00063030-200014020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Delfin J, del Mar Gutierrez M, Gallego-Escuredo JM, et al. Effects of nevirapine and efavirenz on human adipocyte differentiation, gene expression, and release of adipokines and cytokines. Antiviral Res. 2011;91:112–9. doi: 10.1016/j.antiviral.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–18. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prendergast A, Walker AS, Mulenga V, et al. Improved growth and anemia in HIV-infected African children taking cotrimoxazole prophylaxis. Clin Infect Dis. 2011;52:953–6. doi: 10.1093/cid/cir029. [DOI] [PubMed] [Google Scholar]
- 35.Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomized placebo-controlled trial. Lancet. 2004;364:1865–71. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 36.Mulenga V, Ford D, Walker AS, et al. Effect of cotrimoxazole on causes of death, hospital admissions and antibiotic use in HIV-infected children. AIDS. 2007;21:77–84. doi: 10.1097/QAD.0b013e3280114ed7. [DOI] [PubMed] [Google Scholar]