Abstract
This study proposed and evaluated a guideline for outcome evaluation for infants and children with hearing loss who wear hearing aids. The University of Western Ontario Pediatric Audiological Monitoring Protocol (UWO PedAMP) was developed following a critical review of pediatric outcome evaluation tools and was systematically examined by the Network of Pediatric Audiologists of Canada. It consists of tools to gather clinical process outcomes as well as functional caregiver reports. The UWO PedAMP was administered to a clinical population of infants and children with hearing aids. Sixty-eight children were administered the functional outcome evaluation tools (i.e., caregiver reports) a total of 133 times. Clinical process outcomes of hearing aid verification (e.g., real-ear-to-coupler difference) revealed typical aided audibility (e.g., Speech Intelligibility Index). Results for the LittlEARS® questionnaire revealed that typically developing children with hearing loss who wear hearing aids are meeting auditory development milestones. Children with mild to moderate comorbidities displayed typical auditory development during the 1st year of life after which development began to decline. Children with complex factors related to hearing aid use had lower scores on the LittlEARS, but auditory development was in parallel to norms. Parents’ Evaluation of Aural/Oral Performance (PEACH) results indicated no age effect on scoring for children above 2 years of age; however, the effect of degree of hearing loss was significant. This work provides clinicians with a systematic, evidence-based outcome evaluation protocol to implement as part of a complete pediatric hearing aid fitting.
Keywords: outcome measures, outcome evaluation, audiological monitoring, infants, children, hearing loss, hearing aids
Introduction
The primary goal of Early Hearing Detection and Intervention (EHDI) programs is to provide effective intervention by 6 months of age to maximize the infant’s natural potential to develop language and literacy skills (Joint Committee on Infant Hearing [JCIH], 2007). Intervention with hearing aids, as part of a larger intervention plan, is a common choice among families. Audiologists have access to scientifically based strategies and clinical tools to ensure the hearing aids are fitted appropriately to the infant. Outcome evaluation is a recommended component of the pediatric hearing aid fitting process (American Academy of Audiology [AAA], 2003; Bagatto, Scollie, Hyde, & Seewald, 2010; College of Audiologists and Speech Language Pathologists of Ontario [CASLPO], 2002; British Columbia Early Hearing Program [BCEHP], 2006; King, 2010; Modernising Children’s Hearing Aid Services [MCHAS], 2005), however, there is little research related to what a typical outcome might be for an infant who wears hearing aids and how to systematically track the child’s auditory development and performance over time. This may in part be due to the lack, or perceived lack, of well-normed and validated auditory-specific outcome measures available for use with infants and children who wear hearing aids. Several research studies have focused on the overall communication outcomes of children involved in EHDI programs and what factors may affect outcome (e.g., Bass-Ringdahl, 2010; Ching, Dillon, Day, & Crowe, 2007; Moeller et al., 2007a, 2007b; Sininger, Grimes, & Christensen, 2010). These studies reveal important information about the parameters of outcome for children who are early-versus late-identified. For example, these studies show positive effects of early intervention, parental involvement and limiting effects of late identification and inconsistent hearing aid use. Individual clinicians and/or EHDI programs may be inclined to implement some or all of the outcome batteries of such studies when attempting to measure outcomes for individual infants or across programs. Unfortunately, this strategy may not be successful in a nonresearch context: the protocols implemented in these studies were designed for the purposes of research and may have barriers to implementation in clinical practice. These barriers include extensive test batteries that are impractical to administer and score in a typical clinical situation.
The focus of this article is to describe a clinically feasible guideline for monitoring auditory-related outcomes in infants and children, giving equal priority to properties such as normative data, sensitivity, specificity, and reliability as well as to clinical feasibility and utility (Andresen, 2000). Companion articles in this volume include a critical review of existing pediatric outcome evaluation tools (Bagatto, Moodie, et al., 2011) as well as a systematic evaluation of the chosen measures by the Network of Pediatric Audiologists of Canada (Moodie et al., 2011). In the present article, these two sources of information are integrated, and a specific guideline for outcome measurement in a clinical context as well as data for children with hearing loss who wear hearing aids are provided. This guideline is called the University of Western Ontario Pediatric Audiological Monitoring Protocol (UWO PedAMP). The UWO PedAMP is intended to be used with children with permanent hearing loss from birth to age 6 years who wear hearing aids. Audiological monitoring is an important aspect of pediatric audiology whether or not the child has received hearing aids (e.g., the child has unilateral or mild bilateral hearing loss and is not yet aided). The UWO PedAMP can be used for monitoring children who have unaided hearing loss; however, the focus of this article will be on the application of the guideline with children who wear hearing aids.
The investigation reported here was a repeated measures longitudinal intervention study. The purpose of this study was to compare data from a clinical population of infants and children with permanent childhood hearing impairment (PCHI) on a set of outcome evaluation tools to existing norms. Characterization of scores on the tools with infants and children with various audiometric and medical profiles was examined. In this study, children with all degrees and configurations of hearing loss and intervention types as well as those with comorbidities and complex factors (e.g., inconsistent hearing aid use) were investigated. Including these children in this work was unique when compared to the previously mentioned studies that evaluated outcomes in children with hearing loss and no other associated complexities or medical factors. This ongoing work will greatly enhance the understanding of auditory development and performance of a naturally occurring clinical pediatric audiology population.
Method
Guideline Rationale
The UWO PedAMP is an extension of current pediatric hearing aid fitting protocols (e.g., Bagatto et al., 2010) and includes two types of outcome evaluation tools: (a) clinical process outcome measures to characterize the implementation of the previous stages of the hearing aid fitting process (e.g., verification) to aid in the interpretation of functional outcomes and (b) individual patient functional outcome measures in a two-stage process by developmental level. The functional outcome measures are (a) the LittlEARS® Auditory Questionnaire (Tsiakpini et al., 2004) and (b) the Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) Rating Scale (Ching & Hill, 2005a). These measures were chosen based on the results of a critical review (Bagatto, Moodie, et al., 2011) as well as input from pediatric audiologists associated with the Network of Pediatric Audiologists of Canada (Moodie et al., 2011). The questionnaires were deemed to have a high level of evidence and feasibility as described in the companion articles, which supports their inclusion in the UWO PedAMP.
For younger children (see details below), the LittlEARS Auditory Questionnaire is used. For older children, the PEACH Rating Scale is used. Therefore, the tools included in the UWO PedAMP are as follows:
Aided Speech Intelligibility Index (SII) Normative Values Worksheet;
Hearing Aid Fitting Summary;
LittlEARS®Auditory Questionnaire (Tsiakpini et al., 2004; Copyright MED-EL, 2004);
Parent’s Evaluation of Aural/Oral Performance of Children (PEACH; Ching & Hill, 2005a; Copyright Australian Hearing, 2005).
Prior to measuring functional outcomes (LittlEARS and PEACH), summary measures of the hearing aid fitting process are made to characterize that process. These are included in the UWO PedAMP as clinical process outcomes (e.g., Aided Speech Intelligibility Index [SII] Normative Values Worksheet, Hearing Aid Fitting Summary). Hearing aids are used or worn for a trial period by the majority of children who have been identified with PCHI. Evidence-based pediatric hearing aid fitting protocols are followed to positively support the impact of the infant’s hearing aid on his or her ability to develop auditory skills in daily life (e.g., AAA, 2003; Bagatto et al., 2010; BCEHP, 2006; MCHAS, 2005). In the UWO PedAMP, functional outcome evaluation follows the hearing aid verification stage of the fitting process to measure the impact of the fitting. There are two primary reasons to monitor hearing aid fitting process outcomes as part of the UWO PedAMP prior to measuring functional outcomes.
The first reason is to determine whether an individual child’s fitting is providing a typical degree of audibility for a given degree of hearing loss. Clinicians and parents will have a better understanding of how the child is progressing with respect to audiological outcomes when details of the hearing aid fitting are tracked as part of an overall outcome evaluation guideline. For example, if the output of the hearing aid is significantly less than would be typical for other children with similar losses, the child’s ability to use sound for development may be limited relative to a child with a typical fitting.
The second reason for monitoring hearing aid fitting details is at the level of the program as a whole. The brief fitting details gathered in this protocol help to determine, for example, the typical rate at which real-ear-to-coupler difference (RECD) measures are made, or the typical amount of audibility provided by the hearing aids. This information may allow EHDI programs to monitor program-wide clinical process outcomes for such purposes as monitoring protocol use and practice quality.
Clinical Context
The participants in this study were caregivers of children who were seen as part of the Ontario Infant Hearing Program (OIHP). The OIHP is an example of a comprehensive EHDI program that identifies children born deaf or hard of hearing and provides the supports and services they need to develop the language and literacy skills necessary to achieve success in school (Bagatto et al., 2010). The program provides services for children from birth to 6 years of age who are identified with PCHI and their families/caregivers. As well, it monitors those children born with, or who acquire risk indicators for permanent hearing loss throughout early childhood. Program protocols are in place to provide universal newborn hearing screening, audiological assessment, and amplification and communication development services for children found to be deaf or hard of hearing. The OIHP utilizes systematic, evidence-based procedures for hearing aid fitting, including the use of the DSL v5.0a (Scollie et al., 2005), measured RECD values, simulated real-ear verification, and hearing aid orientation.
Every year in the province of Ontario, about 3 in 1,000 babies are either born with a permanent hearing loss or will develop a hearing loss early in their childhood. With a yearly birthrate of approximately 130,000, about 400 babies or preschool children are identified with impaired hearing every year in Ontario. In the fiscal year 2010/2011, 95% of the babies born in Ontario had their hearing screened. In addition, of the 371 children identified with PCHI in 2010/2011, 47 were identified through surveillance of at-risk infants and 173 were from other referral routes (e.g., acquired risk, acquired hearing loss, newly identified) and received an assessment prior to entry into Grade 1. From these routes combined, approximately 2,855 were identified with PCHI (2,252 bilateral, 602 unilateral) from program inception in November 2001 to March 31, 2011. The families of 1,709 of these infants chose hearing instruments, 98 infants wear cochlear implants,1 and the remainder (979) chose neither option or were in the process of obtaining hearing instruments. Reasons for choosing neither option vary and include such factors as opting for manual communication and watchful waiting for children with mild and/or unilateral hearing loss. University of Western Ontario ethics approval was obtained so that five clinicians at four participating clinical sites in Ontario could provide de-identified data. The clinicians were pediatric audiologists with at least 10 years of experience working with infants and young children. Three of the clinics were in the Toronto Region of the OIHP (Humber River Regional Hospital, Markham Stouffville Hospital, Centenary Hospital) where two audiologists collected data and one clinic was in the Southwest Region of the OIHP (University of Western Ontario H.A. Leeper Speech and Hearing Clinic) where three audiologists collected data.
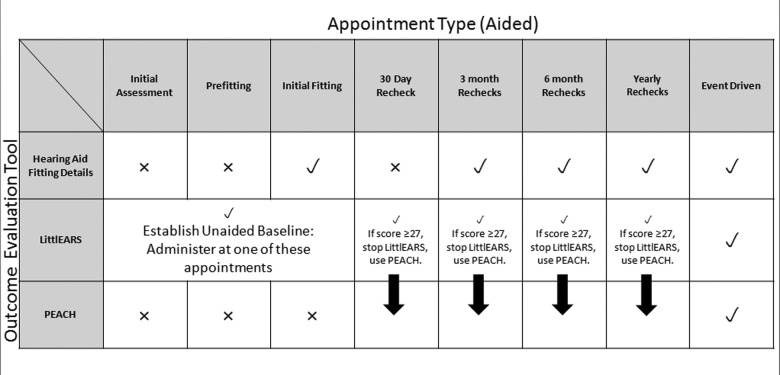
Since April 2010, the UWO PedAMP has been implemented as an extension of the OIHP’s Provision of Amplification Protocol in Ontario, Canada (Bagatto et al., 2010). Facilitating successful clinical implementation of the UWO PedAMP has been an important consideration for the introduction of this guideline in an EHDI program, such as the OIHP. For this reason, a suggested administration timeline is provided to outline when each outcome evaluation tool is used as part of the guideline. The grid in Figure 1 summarizes the administration of each outcome evaluation tool within the UWO PedAMP during a hearing-impaired child’s routine follow-up.
Figure 1.
Administration guidelines for children with PCHI who wear hearing aids
Note: The top row specifies the appointment type and the far left column indicates the outcome evaluation tool within the UWO PedAMP that should be administered. Within the grid, “✓” and “X” designates when an outcome evaluation tool should or should not be administered at a particular appointment.
Each outcome evaluation tool within the UWO PedAMP is listed down the left-hand side of the figure. The clinicians involved in this study were able to determine whether a tool should (“✓”) or should not (“X”) be administered during the specific appointments listed across the top of the figure. Each tool within the UWO PedAMP was administered during a routine clinical appointment.
Participants
Participants included 352 caregivers of infants and children with various audiometric and medical profiles (mean age = 21.7 months; age range = 1.3 to 107.1 months). Eighty-six children were from the Toronto Region of the OIHP and 266 children were from the Southwest Region of the OIHP. Of the total children, 223 had normal hearing and 129 had permanent hearing loss. The purpose of including the normal hearing children was to evaluate existing normative values and clinical feasibility of the tools. Hearing losses ranged from mild to profound and were unilateral (n = 35) or bilateral (n = 94) sensorineural (n = 84) or permanent conductive (n = 18). Twenty-seven children in this sample had auditory neuropathy spectrum disorder (ANSD) and were not fitted with hearing aids at the time of inclusion in the study. Sixty-eight of the children with PCHI were fitted with hearing aids and 61 had no hearing aids at the time of inclusion. Thirty-three of the children with hearing aids were from Humber River Regional Hospital, 18 were from Markham Stouffville Hospital, 6 were from Centenary Hospital, and 11 were from the H.A. Leeper Speech and Hearing Clinic at UWO.
Children with hearing aids had hearing losses ranging from mild to profound, unilateral or bilateral sensorineural (pure tone average = 48.41 dB HL; range = 16.67 to 110.00 dB HL; see Table 1).
Table 1.
Number of Children With PCHI Who Wear Hearing Aids by Hearing Loss Category (dB HL) and Outcome Evaluation Toola
| Degree of PCHI | LittlEARS Data | PEACH Data | Number of Children | Number of Administrations |
|---|---|---|---|---|
| Mild (between 20 and 40 dB HL) | Bilateral = 11 Unilateral = 1 | Bilateral = 15 Unilateral = 1 | Bilateral = 24 Unilateral = 1 | 38 |
| Moderate (between 41 and 55 dB HL) | Bilateral = 18 Unilateral = 0 | Bilateral = 18 Unilateral = 0 | Bilateral = 24 Unilateral = 0 | 51 |
| Moderately-severe (between 56 and 70 dB HL) | Bilateral = 9 Unilateral = 1 | Bilateral = 10 Unilateral = 1 | Bilateral = 14 Unilateral = 1 | 34 |
| Severe (between 71 and 90 dB HL) | Bilateral = 0 Unilateral = 1 | Bilateral = 0 Unilateral = 1 | Bilateral = 0 Unilateral = 1 | 4 |
| Profound (91 dB HL or greater) | Bilateral = 2 Unilateral = 0 | Bilateral = 2 Unilateral = 0 | Bilateral = 3 Unilateral = 0 | 6 |
| Number of Children | 43a | 48a | 68 | |
| Number of Administrations | 58 | 75 | 133 |
Some children have multiple data for the LittlEARS, the PEACH, or both that are not presented here.
In this population study, children with comorbidities and complex factors were included as well as typically developing children. Comorbidities included medical issues such as Down Syndrome, cerebral palsy, and genetic syndromes. Children in this study were identified as having a comorbidity based on clinician report. Children with comorbidities comprised approximately 12.5% (n = 44) of the total sample. Of the 68 children fitted with hearing aids, 32.35% (n = 22) had comorbidities. Complex factors included nonmedical complicating issues that may affect hearing aid outcome such as inconsistent hearing aid use and delayed hearing aid fitting. Approximately 33.82% (n = 23) of the hearing impaired children with hearing aids had complex factors in this sample. This left 33.82% (n = 23) typically developing children from the total sample of children with hearing loss who wear hearing aids.
The following sections provide an overview of the tools included in the UWO PedAMP: the Aided Speech Intelligibility Index (SII) Normative Values Worksheet, Hearing Aid Fitting Details, the LittlEARS, and the PEACH questionnaires. Information about where to locate the different tools within the UWO PedAMP as well as score sheets for the LittlEARS and PEACH questionnaires can be found in the Appendix. Data from the use of these tools will be presented within a large-scale study in which the UWO PedAMP was administered during routine clinical practice.
Clinical Tools
Hearing aid fitting details
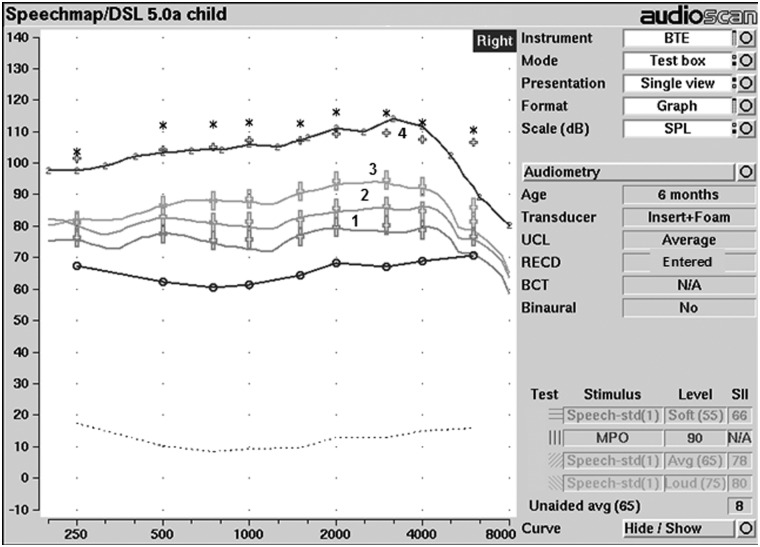
As part of the UWO PedAMP, two tools are provided to monitor and assess the clinical process of hearing aid fitting and include (a) Aided SII Normative Values: Birth to 6 Years Worksheet and (b) the Hearing Aid Fitting Summary (see Appendix). Used together, they provide helpful information for the audiologist, caregivers, and health policy makers about the hearing aid fitting as part of this outcome evaluation guideline. The UWO PedAMP is an extension of the hearing aid fitting process and assumes that the audiologist has followed preferred practice guidelines for pediatric hearing assessment and the fitting of hearing aids to infants and young children (Bagatto et al., 2010). Several steps are followed in the verification stage of the pediatric hearing aid fitting process and include simulated (or predicted) real-ear measurements of hearing aid performance using RECD measurements (Bagatto et al., 2010). Figure 2 displays one example of this procedure that is explained in detail in the protocol (Bagatto et al., 2010).
Figure 2.
SPLogram display of hearing instrument performance in relation to pediatric DSL v5.0a targets for a child with a PTA of 52 dB HL
Note: The solid lines represent the output of the hearing instrument for soft (1), average (2), loud (3) speech inputs and MPO (4) in relation to the various speech targets (large +) and MPO targets (small +). Thresholds (o) and upper limits of comfort (*) are also displayed.
In this guideline, the aim was to minimize the time needed to capture the hearing aid fitting details. For this reason, the exact fit to targets at each frequency and test level was not documented. Instead, the goodness of fit to targets was assessed by the clinician. The overall outcome of the fitting was assessed using three indicators of clinical process: (a) whether the RECD was measured, predicted, or entered from previous file data; (b) whether the clinician measured the maximum power output (MPO); and (c) the amount of audibility provided for low and moderate level speech (via the aided SII).
For both individual-level and program-level outcome evaluation, it was of interest to know whether the RECD was individually measured or predicted. Individually measured RECDs are more desirable for hearing aid fitting than predicted RECD values due to the substantial between-subject variability noted in RECD measures in infants and young children (Bagatto, Scollie, Seewald, Moodie, & Hoover, 2002). Although age appropriate, currently available predicted RECD values only provide a gross estimate of actual RECD values in the pediatric population (Bagatto et al., 2002). Therefore, current pediatric hearing aid fitting protocols require the audiologist to attempt a measurement of the RECD to individualize the fitting for the patient (e.g., Bagatto et al., 2010). It was therefore of interest to know if the RECD was individually measured or predicted. To understand practice fidelity and clinical process outcomes, the clinician therefore indicated whether the RECD was measured or predicted for each ear. Also, if an RECD was measured on one ear and applied to the other ear, or previously measured values were used, these options were available (Table 2). Since the MPO is measured using a narrowband signal and not speech, there is no associated speech audibility index value (i.e., Speech Intelligibility Index) provided. Therefore, the clinician indicated whether or not the MPO was measured during the child’s hearing aid fitting and any follow-up visits. For outcome evaluation of the individual child, this simply documents that this important step was fulfilled (Table 2). At the program level, this information can be used to evaluate programwide adherence to the recommended protocol. For many pediatric hearing aid fitting protocols, measurement of the real-ear aided response (REAR) for low and moderate speech inputs are required (e.g., AAA, 2003; Bagatto et al., 2010; BCEHP, 2006). Since hearing aid verification systems provide an associated SII value for all REARs, the next step was to document the SII values. Including the SII for low and moderate speech in the outcome evaluation process provided information about how typical the hearing aid fitting was for each ear for a particular patient. A complete clinical process outcome measure for the SII included a value from zero to 100 for low- (55 dB SPL) and moderate-level (65 dB SPL) speech inputs. In summary, two SII values per hearing aid fitting were tracked (see Table 2).
Table 2.
Summary of Hearing Aid Fitting Details
| Hearing Aid Fitting Detail | Data to be Tracked (For each aided ear) |
|---|---|
| Real-ear-to-coupler difference (RECD) | Measured Predicted Other ear values Previously measured |
| Maximum Power Output (MPO) | Measured (yes/no) |
| SII for Soft Speech input (55 dB SPL) | Value from 0 to 100 |
| SII for Average Speech input (65 dB SPL) | Value from 0 to 100 |
The SII is a value representing the proportion of speech that is audible to the listener through his or her hearing aids (American National Standards Institute [ANSI], S3.5, 1997). It is an electroacoustic measure, not a behavioral prediction of speech recognition. The SII provides a value that clinicians, caregivers, and teachers can use to conceptualize the proportion of speech that is available to the child. SII values are provided from hearing aid verification systems (e.g., Audioscan Verifit®, Interacoustics Affinity®). If a clinician performs speech-based real-ear verification of the young child’s hearing aids, the SII is computed for each input level tested. For example, in Figure 2, the measured real-ear performance of the child’s hearing aids for an average speech input provides an associated SII value, which indicates that 78% of moderate-level speech is audible to the wearer. The clinician will also be provided with SII values for verification measures made with other speech input levels. In this example, 66% of soft speech is audible.
Recently, normative data for fit to Desired Sensation Level (DSL) Method version 5.0a targets have become available (Moodie, 2009, 2010). These were derived from pediatric fit to target data from 161 ears. The fittings ranged from 1 dB below to 4 dB above the prescribed target on average from 250 to 4000 Hz. From these data, the SII values were extracted to analyze the relation between SII and unaided pure tone average (PTA) hearing threshold levels, using a linear regression (see Figure 3). The results indicated that aided SII values decrease from 100% to 40% as hearing level increases from 20 dB HL to 90 dB HL. Within this range, the data vary by approximately 30% in more than 95% of fittings. This trend is due to the application of the level distortion factor within the SII calculation and narrower bandwidth typical of higher gain fittings (ANSI, S3.5, 1997). Above 90 dB HL, there was too little data to establish a clear trend. Within the UWO PedAMP guideline, this trend was used, as well as the 95% confidence interval surrounding it, to determine whether a given fitting was considered typical for that PTA hearing loss. The Aided SII Normative Values Worksheet was developed for this purpose (see Appendix). Due to the lack of data in the region above 90 dB HL PTA, a typical trend for SII values in this region is not provided. The norms on the worksheet can therefore be used clinically to conceptualize audibility after some fit to target criteria (e.g., within 5 dB for losses with a PTA ≤70 dB HL) have been established.
Figure 3.
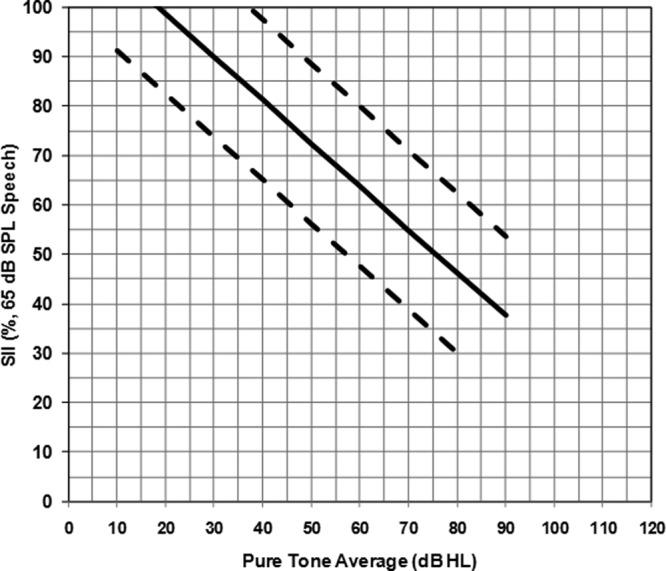
Graph from the Aided SII Normative Values Worksheet displaying SII values for a 65 dB speech input
Note: The regression line was obtained from hearing aid fittings on 161 ears of infants and children. The solid line represents the linear fit to the data and the dashed lines represent the upper and lower 95% confidence interval ranges. An SII value that falls between the dashed lines is considered to be typical audibility for that pure tone average.
Within the context of the OIHP, all clinicians within the program received training on measurement of all of these indicators, and other mechanisms within the program allow for specific file audit to look at practice quality in detail. The main interest, therefore, lies in the protocol elements present in a given hearing aid fitting, or across hearing aid fittings programwide, as a means of either (a) measuring how often clinicians employ these protocol elements and/or (b) having a means to characterize cases in which protocols were followed versus not followed.
Reporting hearing aid fitting details
To facilitate the collection of relevant hearing aid fitting details, the UWO PedAMP provides a Hearing Aid Fitting Summary form (see Appendix). This form provides a way of recording, at regular intervals, important information about the hearing aid fitting, such as the details of the RECD measurement, the SII values associated with low and moderate level speech inputs, and whether an MPO measurement was made. These clinical process variables were recorded at the initial hearing aid fitting and at routine 3-month, 6-month and yearly follow-up visits (see Figure 1). Hearing aid fitting details were also recorded in event-driven situations.
The LittlEARS Auditory Questionnaire
The LittlEARS Auditory Questionnaire is a caregiver-report functional outcome evaluation tool. It is included in the UWO PedAMP for evaluation of younger children, as discussed below. According to the authors, the purpose of the LittlEARS Auditory Questionnaire is to assess the auditory behavior of infants with PCHI who wear hearing aids or cochlear implants (Coninx et al., 2009; Tsiakpini et al., 2004, Copyright MED-EL, 2004). The 35 items in the LittlEARS assess auditory development during the 1st 2 years of hearing in the real-world and tap into receptive and semantic auditory behavior as well as expressive-vocal behavior. The questions are listed in an age-dependent order and are in a yes/no format. The total of all “yes” answers provide a score that can be compared to average and minimum age-dependent values. These values are provided in 1-month age categories based on normative data (Coninx et al., 2009). The LittlEARS is designed to be answered by caregivers and is not affected by how it is administered (i.e., under professional guidance or independently). It has been suggested that using a caregiver observation tool at the early stages may be helpful to caregivers who are starting to navigate through the world of hearing loss and hearing aids (Harrison, 2000). The LittlEARS supports this function for caregivers because the items provide examples that introduce them to early auditory behaviors and prepares them to understand what auditory behaviors can be observed at later stages of development.
A validation study of the LittlEARS questionnaire was conducted on 218 normal hearing children from German-speaking families (Coninx et al., 2009). Results indicated that the questionnaire is reliable (split half r = .88), has good internal consistency (Cronbach’s α = .96), and predictive accuracy (Guttman’s λ = 0.93). There is also high correlation between the overall score and the age of the children (r = .91). The data collected from the caregivers were used to obtain normative values for the development of early auditory behavior in normal hearing infants and used to derive average and minimum values for scoring. A validation study was conducted with 63 children in Germany and Italy who wear cochlear implants. The results indicated that the LittlEARS questionnaire is appropriate for use with children provided with cochlear implants early in life and the results can be compared to the normative data (Kuehn-Inacker, Weichbold, Tsiakpini, Coninx, & D’Haese, 2003). Currently there is a validation study being conducted in the United States with English-speaking infants who wear cochlear implants (www.ClinicalTrials.gov Identifier NCT00785707). The questionnaire has also been validated in 15 different languages with families of normal hearing infants and toddlers up to 24 months of age (Bagatto, Brown, et al., 2011; Coninx et al., 2009). Regression curves for each language were essentially equivalent to the German-derived norms.
Further review of the feasibility of the LittlEARS questionnaire in clinical practice indicated that changes to the score sheet would facilitate its use with children who experience developmental delay (Moodie et al., 2011). For this reason the score sheet shown in the Appendix was developed. This tool maintains the original normative trajectory and cutoff scores but extends the age range that may be plotted. This revised score sheet is included as part of the UWO PedAMP and was considered a useful addition to the guideline by the Network of Pediatric Audiologists of Canada (Moodie et al., 2011).
Parents’ Evaluation Of Aural/Oral Performance Of Children
The Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) Rating Scale is included as a caregiver-report functional outcome evaluation tool for use after the LittlEARS questionnaire is no longer appropriate. The PEACH in its original diary form is conducted using a structured interview format and has questions that address quiet and noisy situations as well as hearing device and telephone usage (Ching & Hill, 2005b). The PEACH Diary requires caregivers to observe their child for at least 1 week and record their observations for the 13 scenarios over that time period. They are also asked to rate the frequency of each behavior and provide examples of when the child did or did not exhibit a particular response. After the observation period, the audiologist meets with the caregiver to address each item in a face-to-face interview. The interview is structured to solicit detailed information from the caregiver, rather than yes/no answers. Normative data for the PEACH were obtained from 90 parents of normal hearing children and 90 parents of children with PCHI who wear hearing aids (Ching & Hill, 2007). The tool demonstrated good internal consistency (Cronbach’s α = 0.88) and high test-retest reliability (r = 0.93). Normal hearing children (age range = 0.25 to 46 months) demonstrated an increase in performance from about 6 months of age and close to perfect performance (i.e., 90%) was achieved by about 3 years of age. Children with increasing hearing loss showed a decrease in performance (age range = 4 months to 19 years). Descriptive statistics for the PEACH were also reported indicating an overall test mean of approximately 62% for children with PCHI, with similar mean scores for the quiet and noise subscales. The authors noted that the children with hearing loss were late-identified, and the functional performance of children who are early identified may be improved (Ching & Hill, 2007). A follow-up study with children with severe-to-profound hearing loss demonstrated that the PEACH is sensitive to changes in frequency response slopes in hearing aids (Ching, Hill, & Dillon, 2008).
The observation and interview process required for the PEACH Diary was found to be heavy in administrative and respondent burden as reported by the Network of Pediatric Audiologists of Canada (Moodie et al., 2011). Specifically, the time it takes to administer and score the PEACH Diary is longer and more involved compared to the PEACH Rating Scale. In addition, literacy barriers for some families may prevent completion of the PEACH Diary due to the independent nature of the diary version. These limitations were reflected in the PEACH Rating Scale being rated more favorably in the critical review (Bagatto, Moodie, et al., 2011) and accepted by a higher percentage of participants in the Network (Moodie et al., 2011) compared to the PEACH Diary. In addition, as reported in a research study (Golding et al., 2007) the caregiver’s ability to observe their child may have varied and may have been limited by competing factors in the household (i.e., number of children, wellness of the child, lifestyle; Golding et al., 2007). Also, an inexperienced interviewer may have had difficulty extracting useful examples from the caregivers even though the interviewer received instructions on how to administer the PEACH (Golding et al., 2007).
A Rating Scale version of the PEACH (Ching & Hill, 2005a) has been made available and includes most of the scenarios from the original PEACH Diary (Ching & Hill, 2005b). The PEACH Rating Scale (referred to as the PEACH for the remainder of this article) appears to be more acceptable by clinicians and caregivers because the respondent and administrative burden have been reduced (Moodie et al., 2011). The instructions ask caregivers to recall their child’s behavior in everyday life over the past week and rate their child’s hearing performance across a range of hearing and communication scenarios. The nature of the rating scale allows it to be answered by the caregiver during an appointment with guidance from the clinician, reducing respondent and administrative burden (Bagatto, Moodie, et al., 2011). Therefore, the PEACH was selected for use in the UWO PedAMP, with older infants and children who have attained ceiling performance on the LittlEARS Auditory Questionnaire. Ceiling performance on the LittlEARS occurs when the minimum score of 27 or greater has been achieved. This facilitates the use of the LittlEARS with children of various developmental trajectories by providing a stopping rule based on score and not by chronological age before moving to the PEACH. Also, items on the LittlEARS display similar content as the PEACH around Item 27. Therefore, for children involved in this study, the LittlEARS was administered until the child reached a ceiling score of 27, regardless of age. Then, the PEACH was administered at the next routine follow-up appointment. The modified administration guidelines for both the LittlEARS and the PEACH based on the results of this study are outlined in the discussion section of this article.
Results
Hearing Aid Fitting Details
The RECD and MPO were both reported for 75.0% of the children involved in this study. The RECD was measured 56.8% of the time and predicted values were used 27.5% of the time. Reasons for using predicted values were most often due to excessive cerumen in the ear canal or a very active child. RECD values from the other ear were used for the ear with the better PTA 5.9% of the time. Previously measured values were used 9.8% of the time.
SII values for soft speech inputs were reported for 62 out of 68 children (91.2%) with PCHI who wear hearing aids in this study. These SII values had an average percentage of 66.2 (range = 11 to 96). For average speech inputs, 64 out of 68 SII values (94.1%) were reported for children with hearing aids. Percentages were 74.9% on average for these SII values (range = 21 to 97). The SII values for average speech have been plotted within the Aided SII Normative Values Worksheet by degree of hearing loss (Figure 4). It can be seen that for the children involved in this study, the majority of the SII values for average speech are considered to be typical for the degree of hearing loss.
Figure 4.
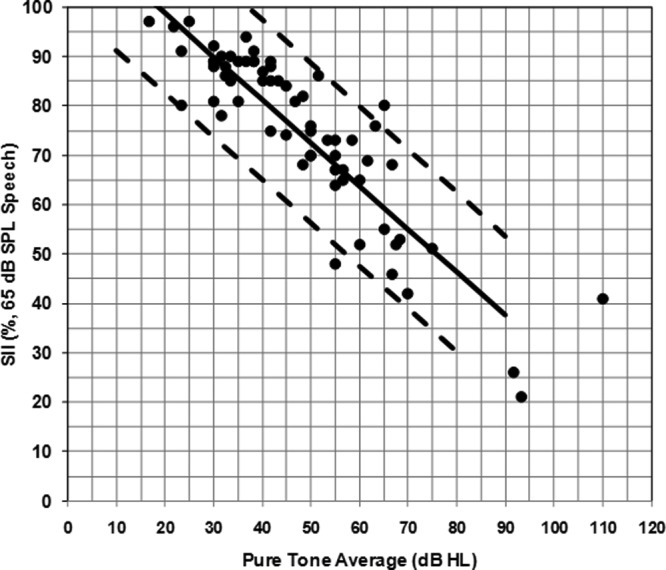
SII values for average speech inputs by PTA for children with hearing aids involved in this study (filled circles)
Note: Solid and dashed lines are from the Aided SII Normative Values Worksheet. The solid line is the average SII normative values and the dashed lines are the upper and lower 95% confidence interval ranges.
LittlEARS Data From Children With Hearing Loss Who Wear Hearing Aids
Of the total participant sample, 43 caregivers of children (mean age = 27.3 months; age range = 6.9-72.7 months) with PCHI who wear hearing aids were administered the LittlEARS a total of 58 times. Twenty-eight children received a single administration, and 15 children received repeated administrations, ranging in number from two to five longitudinal repetitions. Many of the children in this sample were identified as having comorbidities (39.5%; n = 17) and complex factors (32.6%; n = 14). A total of 27.9% of children (n = 12) in this LittlEARS sample were typically developing and had no complex factors related to amplification (see Figure 5).
Figure 5.
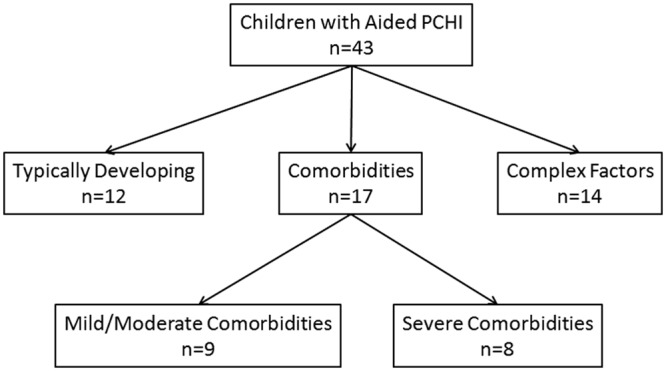
Subgroup flowchart for children with hearing aids whose caregivers were administered the LittlEARS Auditory Questionnaire
Note: Of the total sample with hearing aids, these children were grouped into those with typical development, comorbidities, and complex factors.
Children with comorbidities included those who were premature (i.e., born 37 weeks gestational age relative to a 40-week term) as well as those with other medical issues beyond PCHI. These children were further separated into a group with mild to moderate comorbidites (n = 9) and a group with severe comorbidities (n = 8). Children with severe comorbidities were born full-term and were indicated by the clinician to have a severe manifestation of a disorder or a syndrome causing multiple issues that could potentially interfere with auditory performance.
Caregivers’ responses on the LittlEARS indicated that children with severe comorbidities were not meeting auditory development milestones for their age and their individual scores were less than 27 out of 35, regardless of age (see Figure 6). Given the small sample size and therefore low power in this group (Lee, 2004), these data were not subjected to further analysis. More data will be obtained to further characterize this important subpopulation. Children with mild to moderate comorbidities were analyzed as a separate group.
Figure 6.
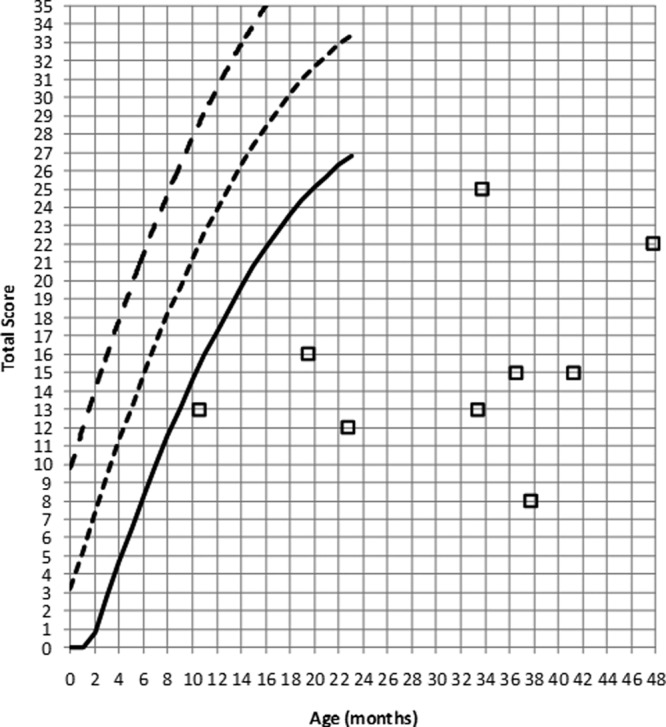
LittlEARS scores from children with hearing aids who were born full-term and have severe comorbidities
Note: The solid line indicates the minimum expected score, the small dashed line indicates the average expected score and the large dashed line indicates the maximum expected score from the German-derived norms. Open squares indicate LittlEARS scores from children with PCHI who have severe comorbidities in this sample. Children with scores above the solid line are considered to be meeting auditory development milestones for their age and children with scores below the solid line are considered to not be meeting milestones.
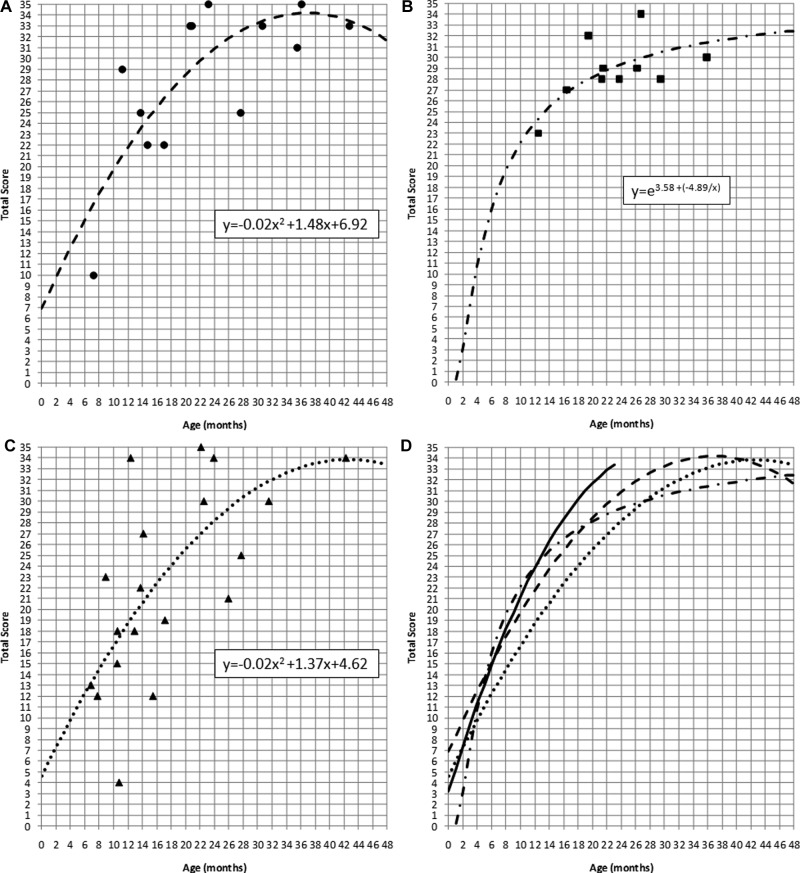
The LittlEARS scores for the remainder of the children were grouped into the following categories prior to analyses: (a) typically developing; (b) mild to moderate comorbidities; and (c) complex factors. Regression analyses were conducted on each group separately to characterize the cross-sectional trajectory of scores by age, per group. For children who were typically developing, a quadratic regression curve provided the best fit to the data (R2 = 0.60; F = 8.20, df = 13, p <0 .01): this was the curve type used with the validation data from the normative study for this questionnaire (Coninx et al., 2009). The regression equation and the quadratic curve fit to the data can be found in Figure 7a. The scores from the children with mild to moderate comorbidities were best fitted with an s-shaped function (R2 = 0.62; F = 18.27, df = 13, p < 0.01), with the regression equation and curve fit noted in Figure 7b. Finally, the scores for children with complex factors were fitted using a quadratic regression curve, as seen in Figure 7c (R2 = 0.43; F = 7.26, df = 13, p < 0.01). Comparing the regression lines from each subgroup to each other as well as to the normative values (Figure 7d) indicates that children who are typically developing are generally meeting auditory development milestones across age. Children with mild to moderate comorbidities show typical auditory development up to about 12 months of age where their scores begin to decline compared to normative data. Finally, children with complex factors associated with hearing aid use appear to be performing in parallel, but have lower scores, compared to typically developing children without complex factors.
Figure 7.
LittlEARS scores (y-axis) by age (x-axis) and regression lines from children with hearing aids who (a) are typically developing and have no comorbidities or complex factors (filled circles); (b) have mild to moderate comorbidities (filled squares); and (c) have complex factors (filled triangles)
Note: The various lines indicate the regression for each set of data: (a) large dashed; (b) dotted-dashed; and (c) small dashed. Regression equations are noted within each figure. The fourth panel (7d) displays all regression lines on a single graph and compares them to the average normative values (solid line).
PEACH Data From Children With Hearing Loss Who Wear Hearing Aids
Forty-eight caregivers of children with PCHI who wear hearing aids were administered the PEACH a total of 75 times. Twenty-eight children received a single administration, and 20 children received two to five repeated administrations of the PEACH. Of the children involved, 29.2% (n = 14) were born 37 weeks gestational age or earlier relative to a 40-week term and/or had other identified medical issues besides hearing loss (i.e., comorbidities). In addition, 37.5% (n = 18) of the children were noted to have a complex factor related to amplification (i.e., inconsistent hearing aid use, delayed fitting due to late identification or other factors). The remaining 33.3% (n = 16) children were full-term, typically developing, early identified, enrolled early in programs of intervention, and did not have complex factors related to amplification.
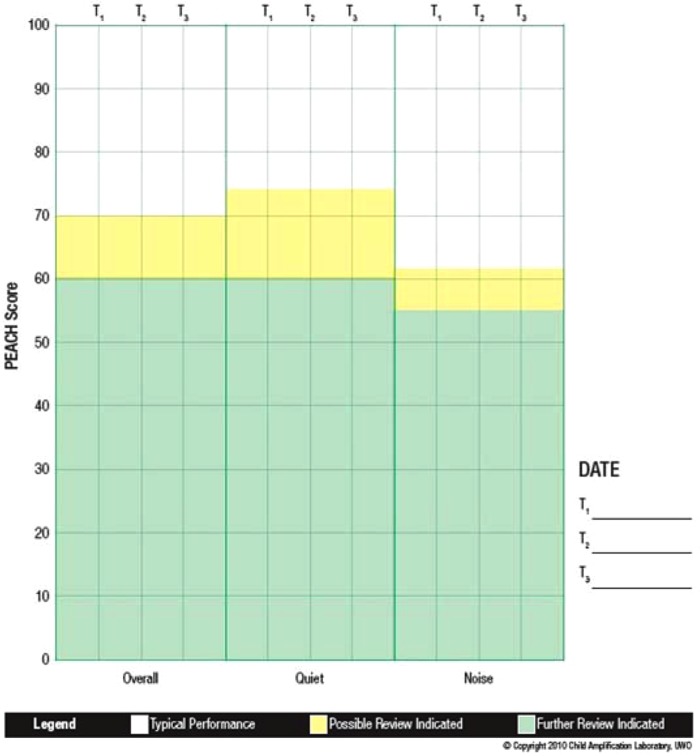
Descriptive statistics are reported on the PEACH score sheet (see Appendix) for children who are typically developing (Figure 8). The average overall score was 84.5% (SD = 11.04) and the quiet and noise subscales were 86.0% (SD = 12.65) and 82.3% (SD = 12.94), respectively. This indicates that children who were identified and fitted early with high-quality amplification and who are typically developing achieve high scores on the PEACH. In fact, the scores of children with hearing aids in this sample are approaching the high score of 90% achieved by normal hearing children by age 3 years.
Figure 8.
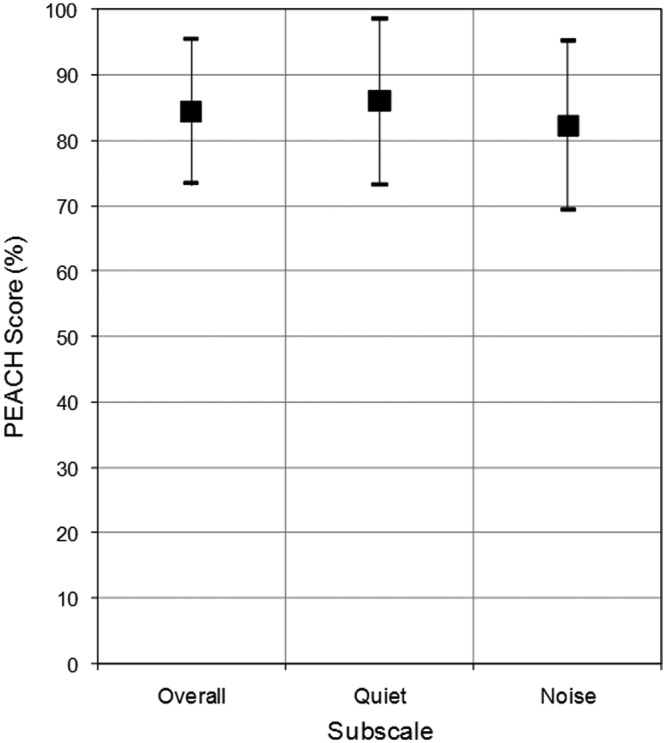
PEACH scores from typically developing, full-term children with hearing aids
Note: Squares represent average percentage scores for each subscale and vertical bars represent the standard deviation around the mean. Note that all scores are within the “Typical Performance” (nonshaded) range for this sample of children.
Analysis 1
The total sample of children were grouped into the following categories prior to regression analyses: (a) typically developing; (b) those with mild to moderate comorbidities; and (c) those with complex factors. There were no children in this sample with severe comorbidities as described in the LittlEARS results section. Regression analyses were conducted on each group separately. For all children who were typically developing, an s-shaped curve provided the best fit to the data (R2 = 0.13; F = 4.36, df = 30, p < 0.05), where the dependent variable was the overall PEACH score and the independent variable was age in months. The regression equation and the s-shaped curve fit to the data can be found in Figure 9. It can be noted that there were approximately 5 children under the age of 24 months included in this analysis, which may have contributed to the significant s-shaped regression curve. Recall that the UWO PedAMP functional outcome evaluation tools were administered using a two-stage process by developmental level. The LittlEARS has a suggested age range of birth to 24 months but this was adjusted to use a score-based stopping rule within the UWO PedAMP for this study because some of the items on the PEACH were considered to be beyond the developmental range of children younger than 24 months. Therefore, the young children were removed and a regression analysis was repeated on typically developing children older than 24 months. The result of this analysis was a nonsignificant linear regression (R2 = 0.009; F = 0.02, df = 25, p > 0.05; Figure 9). This provides support to the idea that the PEACH may be used for children who are typically developing and older than 24 months without the need for age-corrected scoring. A comparison of the curves plotted in Figure 9 indicate that there is no significant age effect on overall PEACH scores after 24 months of age, which supports using the PEACH questionnaire for children older than 24 months of age.
Figure 9.
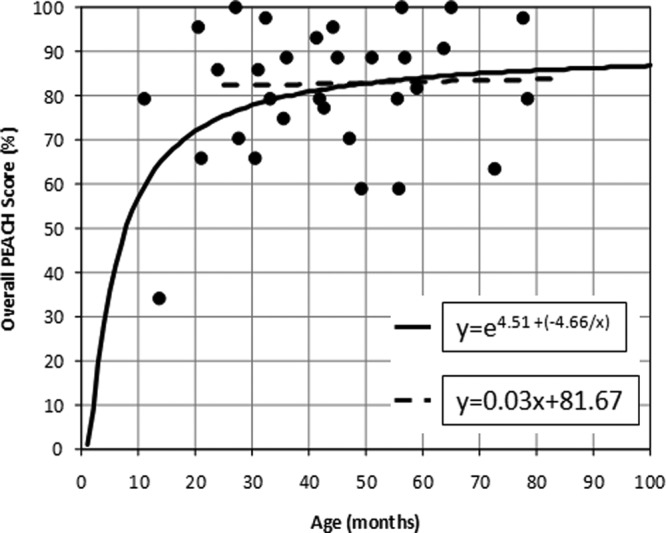
PEACH scores (y-axis) by age (x-axis) and regression lines from typically developing children (filled circles) with hearing aids
Note: The solid line is an s-shaped regression for typically developing children of all ages involved in this study. A nonsignificant linear regression is shown with the dashed line for typically developing children over the age of 24 months. Regression equations are noted in the figure.
Analysis 2
A multivariate analysis of covariance (MANCOVA) was conducted to determine the impact of degree of hearing loss and complexity (e.g., comorbidities and complex factors combined) on the scores for the PEACH quiet and noise subscales. With complexity as the independent variable and the degree of hearing loss as the covariate, results indicated that the multivariate effect of degree of hearing loss was significant, F(2, 54) = 5.713, p < 0.05, η2 = 0.175, but complexity was not, F(2, 54) = 1.643, p > 0.05, η2 = 0.057. Univariate effects confirmed that children who are typically developing or have complexities did not differ on their PEACH scores for either the Quiet, F(1, 55) = 2.366, p > 0.05 and Noise, F(1, 55) = 3.163, p > 0.05, subscales. However, the degree of hearing loss was found to have a significant impact on PEACH scores for both the quiet, F(1, 55) = 11.473, p < 0.05 and noise, F(1, 55) = 4.177, p < 0.05 subscales.
Discussion
This intervention study evaluated pediatric outcome evaluation tools chosen for the UWO PedAMP to assess auditory development (LittlEARS) and auditory performance (PEACH) in children with PCHI who wear hearing aids. Auditory-specific outcomes are one way to measure how well a child with PCHI is performing with his or her hearing aids. It is also important to consider overall communication outcomes, including speech- and language-based outcomes. However, the current work focused on auditory-specific outcomes. In addition to these functional outcomes, clinical process outcomes were assessed by tracking hearing aid fitting details using clinical tools. This important aspect of the UWO PedAMP provided a description of the hearing aid verification process without the need to report fit to target details but by using the SII to provide a gross index of a typical fit to target for the child’s PTA. The clinical process tools provided useful information for the interpretation of the functional outcomes measured by the LittlEARS and the PEACH questionnaires. The majority of hearing aid fitting details were reported and values reflected good hearing aid verification process. Evaluation of the LittlEARS with children with hearing aids indicated the typically developing children in this sample were meeting auditory development milestones across age. Children with mild to moderate comorbidities showed typical auditory development during the 1st year of life then showed a decline in scores compared to existing norms for normal hearing children. Children with severe comorbidities were too small of a sample to conduct an analysis, but more data collection will help to further characterize this group. Children with complex factors related to hearing aid use appeared to have lower scores compared to normal hearing children but did show the same rate of improvement across age. The PEACH results indicated no effect of age on auditory performance as shown by a nonsignificant trend for typically developing children above the age of 24 months. Further analysis indicated that the degree of hearing loss affects scores on the PEACH but complexity does not.
Limitations of this study include the fact that the pediatric audiologists involved in this work had several years of experience with fitting hearing aids to infants and young children. Including an outcome evaluation guideline in their routine practice may have been more of a challenge had the clinicians not been familiar with strategies used in the prior stages of the hearing aid fitting process (e.g., RECD measures, simulated real-ear verification procedures). Therefore, extending the hearing aid fitting process to include the UWO PedAMP was likely less of a barrier for daily clinical practice for the audiologists involved in this study. In addition, the clinicians had the support of the OIHP and regional coordinators to add outcome evaluation tools to their regular clinical routine. The clinicians reported that the UWO PedAMP takes approximately 15 to 20 min of extra clinical time including working with the parents and completing forms for the patient’s chart. This may be a barrier in some clinics where time is limited and clinical managers do not see the importance of measuring outcomes of infants and children who wear hearing aids. One final limitation of this study is the sample size and the fact that children with comorbidities and complex factors were included as study participants. Of the 68 children in the study with hearing loss who wear hearing aids, a total of 23 were typically developing. This was further divided into 12 typically developing children with LittlEARS data and 16 typically developing children with PEACH data (many had repeat administrations). These numbers are approaching the suggested sample size of 20 (Lee, 2004) for each group, however, at this point, the current sample size for each questionnaire may be insufficient to draw firm conclusions about the functional performance of typically developing children who wear hearing aids.
Through this work, clinical administration guidelines were developed to improve the feasibility and potential clinical implementation of the guideline used in this study. This work is unique compared to other outcomes studies in that the guideline implemented here was designed for clinical use and not solely for the purposes of research. Therefore, a focus on reducing barriers to implementation in clinical practice was an important aspect of the development of the UWO PedAMP (Moodie et al., 2011). As such, children with other medical issues in addition to hearing loss as well as complex factors related to hearing aid use were included as participants in this study. This may support a better understanding of the clinical application of the LittlEARS and PEACH in a typical clinical population. Also, application of these tools in clinical practice resulted in clinical administration modifications (e.g., extending the age range of administration for the LittlEARS, particularly for children who have developmental delays) and the design of useful score sheets for record keeping and interpretation. These modifications are described below for each functional outcome evaluation tool. Clinical score sheets can be found in the Appendix. In addition, case examples are provided below to illustrate the use of the UWO PedAMP in clinical practice. We hope that the results of this clinical research and subsequent modifications to existing outcome evaluation tools will provide clinicians with a systematic, evidence-based outcome evaluation protocol to implement as part of a complete pediatric hearing aid fitting.
LittlEARS Administration Guidelines
Within the UWO PedAMP, the LittlEARS Auditory Questionnaire can be administered for children with normal hearing as well as for children with hearing loss who wear hearing aids. The LittlEARS uses a simple “yes/no” format and has items that allow a gradual progression through the tool as the child develops. Therefore, it is recommended that all of the questions be answered, regardless of the number of consecutive “no” answers or the child’s hearing aid status. The tool was developed for infants in their 1st 2 years of life, however, the work presented here has revealed that it is also suitable for children older than 2 years of age who may be premature, who present with atypical development, or who are in the early stages of hearing aid use. Therefore, the score sheet was revised to include a wider age range of use with children up to 48 months of (adjusted) age (see Appendix). Further data collection will facilitate the characterization of LittlEARS scores for infants and children with various audiometric profiles for application in a clinical context. For example, when a score is obtained for a child with aided severe PCHI, the clinician will be able to relate that score to data collected from a group of typically developing children with the same aided degree of hearing loss. On the other hand, many of the children in this initial data set have other medical issues or complex factors and these children may ultimately be characterized differently with future data collection.
It is recommended that administration of the LittlEARS occur at some point prior to hearing aid fitting and at regular follow-up visits (see Figure 1 for administration guidelines). If the child is not wearing hearing aids but has an identified hearing loss, the questionnaire may also be useful for monitoring auditory development and tracking progress over time although data supporting this use are not yet available. In this case, the LittlEARS should be administered at every regular follow-up visit. The total “yes” score is entered on the score sheet at the point where age and score meet. A child with a score in the shaded region is considered to not be meeting auditory milestones for his or her age. A child with a score above the shaded region is considered to be meeting auditory development milestones for his or her age. Within the UWO PedAMP, when a minimum score of 27 or better is achieved on this tool, the child’s performance is considered to be at a ceiling. If ceiling is reached and the child is older than 24 months of age, the LittlEARS should no longer be administered. Instead, the clinician can begin to administer the PEACH, either at that appointment or at the next follow-up visit. This modification is supported by the outcome of the LittlEARS Auditory Questionnaire on those children with severe comorbidities and the fact that the items on the questionnaire display similar content as the PEACH around Item 27. This is further discussed in the next section.
PEACH Administration Guidelines
Within the UWO PedAMP, the PEACH may be administered to children with normal hearing as well as to children with hearing loss who wear hearing aids. A comparison of the LittlEARS and the PEACH in terms of developmental range indicates that some items on the PEACH may not be within the developmental abilities of younger infants. Roughly 17 children with moderate to moderately-severe hearing impairment were younger than 50 months of age in the PEACH normative data (Ching & Hill, 2007). Scores from these younger children and their normally hearing peers are lower, with normally hearing children reaching ceiling performance by 3 years of age. While results from this study, as well as others, reveal the PEACH appears to be sensitive to levels of hearing loss, its age-sensitivity may be due to the difficulty of items for younger infants or toddlers. Therefore, in this guideline a two-stage developmental process for administration is recommended: the LittlEARS is administered until a ceiling score and age criteria are met then the PEACH is administered. This is supported by the current PEACH data indicating there is no age effect on scores for children above 24 months of age. Having the parent of a young infant complete the PEACH may be discouraging at the early stages as some questions may not be developmentally appropriate, making it seem as though the child is not performing well (i.e., respondent burden may be too high). Although the authors suggest certain modifications of items for use with young infants, the specific age range for modification is not known. At young ages, the LittlEARS questionnaire includes items that are developmentally appropriate without modification. Therefore, based on the findings of this study the UWO PedAMP guideline has been modified such that administration of the PEACH begins when the child has reached a score of 27 or greater (i.e., ceiling performance) on the LittlEARS Auditory Questionnaire and the child is older than 24 months of age. These prerequisites should help to ensure that the child’s auditory skills are more likely within the range of the PEACH.
An accompanying PEACH score sheet was developed as part of the UWO PedAMP and provides assistance with interpretation of individual scores (Appendix). Results from previous studies of the PEACH as well as the current work have been included on the current version of the PEACH score sheet and can assist with interpretation of individual scores. The unshaded and shaded regions can be used as benchmarks against which to interpret individual scores. As the PEACH is routinely used in clinical practice, the performance ranges on the score sheet will be validated and the results will be incorporated into future versions of the UWO PedAMP as needed.
Providing guidance for administration and interpretation of the tools supports the implementation of an evidence-based clinical guideline for outcome evaluation in the pediatric population. In addition, case examples are suggested as a way to support clinical implementation of the UWO PedAMP beyond the research results of this study (Kassirer, 2010). For this reason, two case examples demonstrating the use of the UWO PedAMP are provided below.
Case Examples
Case Example 1: Michael
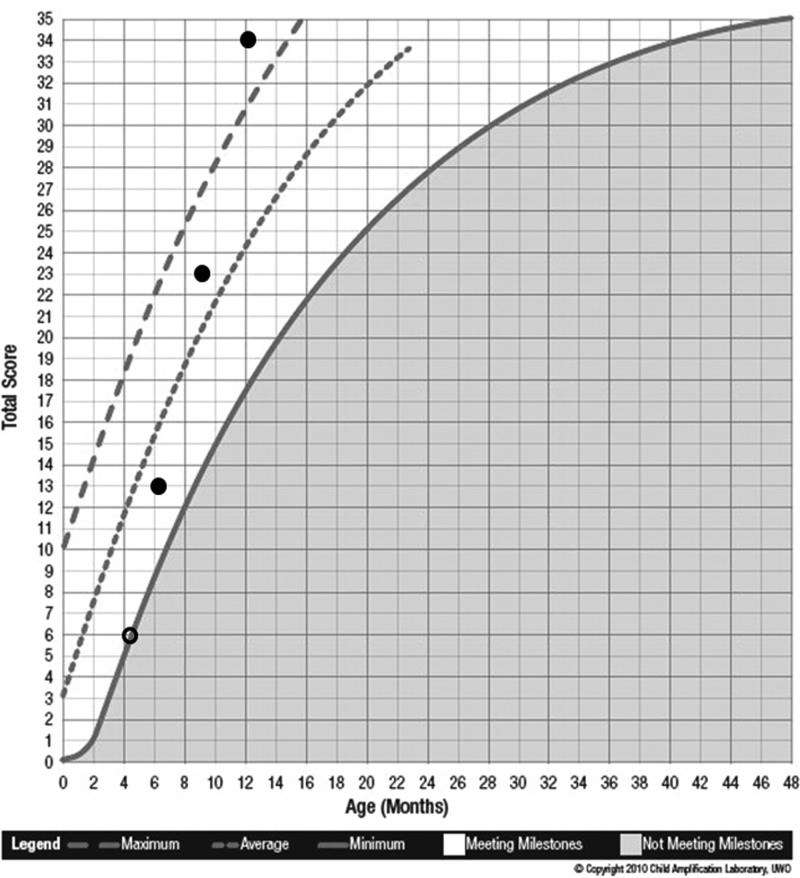
Michael was born full-term without complications with no reported family history of hearing loss. He was identified with a mild sloping to moderately-severe sensorineural hearing loss in both ears (PTA right = 43.3 dB HL; PTA left = 46.6 dB HL) when he was approximately 4 months old. Prior to obtaining hearing aids, Michael’s mother completed the LittlEARS Auditory Questionnaire. The total unaided LittlEARS score was 6. As seen on the score sheet shown in Figure 10, Michael was meeting minimum auditory development milestones for his age without hearing aids. At 5 months of age, Michael was fitted binaurally with hearing aids and the fit to targets were assessed during electroacoustic verification. Hearing aid fitting details were recorded on the Hearing Aid Fitting Summary form. Following a fit to targets assessment, the SII values were transferred to the Aided SII Normative Values Worksheet to visually see whether the child had typical audibility from the hearing aids (see Appendix). In this example, the SII for an average speech input for the right (86%) and left (82%) ears fell within the 95% confidence interval (dashed lines) for Michael’s degree of hearing loss (Figure 11). When compared to aided SII norms, it can be seen that both hearing aids were providing a typical degree of audibility for Michael’s degree of hearing loss for an average speech input. If the SII values fell below the lower dashed line, the values would be considered to be lower than a typical SII for Michael’s degree of hearing loss. If this situation occurred, the clinician could consider modifying the hearing aid fitting to obtain a closer match to targets and thus an improved SII value prior to proceeding with the functional outcome evaluation tools in the UWO PedAMP.
Figure 10.
LittlEARS score sheet for case example: Michael
Note: The solid line indicates the minimum expected score, the small dashed line indicates the average expected score and the large dashed line indicates the maximum expected score from the German-derived norms. Circles represent the LittlEARS Score (y-axis) plotted by the child’s age in months (x-axis). The open circle is the unaided score and the filled circles represent scores in the aided condition. Scores in the nonshaded region indicate the child is meeting auditory development milestones for his age and scores in the shaded region indicate the child is not meeting auditory development milestones for his age. Michael was meeting minimum auditory development milestones for his age prior to being fitted with amplification. While wearing the hearing aids, Michael’s scores improved to where he was showing progress and meeting auditory development milestones for his age.
Figure 11.
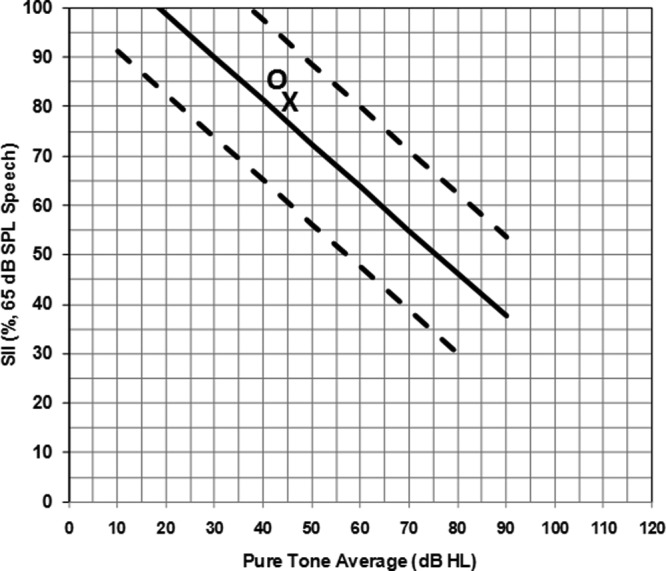
Aided SII values for case example: Michael
Note: SII values (y-axis) for an average speech input are plotted for the right (O) and left (X) hearing aid fittings by Michael’s PTA (x-axis). Since the symbols fall within the 95% confidence intervals (dashed lines), it can be concluded that Michael’s hearing aid fitting is providing a typical degree of audibility for his degree of hearing loss, in both ears.
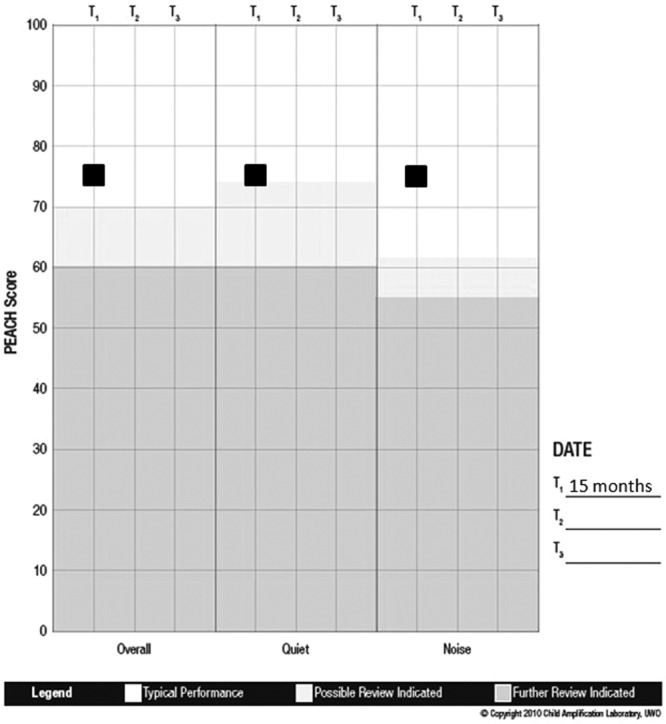
After experience with the hearing aids for 1 month, Michael’s mother completed the LittlEARS questionnaire thinking about Michael’s auditory behaviors while wearing the hearing aids. The score was 13 at approximately 6 months of age, indicating that Michael was meeting typical auditory development milestones for his age in the aided condition (Figure 10). At the 3-month hearing aid follow-up appointments, when Michael was 9 and 12 months of age, he was still meeting auditory development milestones for his age with scores of 23 and 34, respectively, on the LittlEARS (Figure 10). Since Michael’s score on the most recent LittlEARS exceeded a score of 27, which is considered the ceiling score for the UWO PedAMP, the PEACH was administered at his next follow-up appointment. He scored 75% on the overall, quiet, and noise subscales, which is in the target performance range for the PEACH (Figure 12). As discussed above, given that Michael was less than 2 years of age at the time of administration of the PEACH, performance on the tool may improve as he gets older. This example illustrates the result from the group analysis that some children may be too young for the PEACH and scores should be interpreted with caution. For this reason, our current recommendation is that the LittlEARS should be administered until the child is at least 2 years of age and continues to meet the ceiling score criteria.
Figure 12.
PEACH score sheet for case example: Michael
Note: The PEACH percentage scores (y-axis) are plotted within each subscale (x-axis) for this case example. Results indicate that Michael is demonstrating typical auditory performance while wearing the hearing aids.
Michael’s results on the UWO PedAMP indicate that intervention with hearing aids (e.g., clinical process) and supporting communication development intervention resulted in functional outcome evaluation scores that show good auditory development and performance.
Case Example 2: Emma
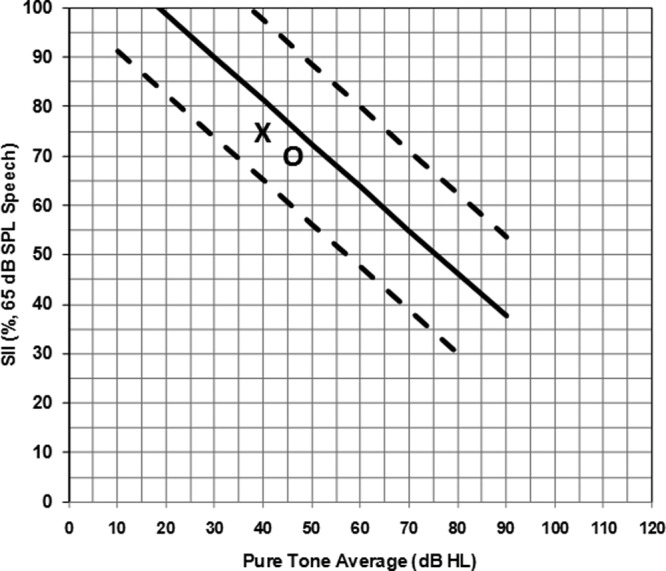
Emma was born full term without complications with no reported family history of hearing loss. She had her hearing screened at birth and did not pass in either ear. Her parents did not pursue follow-up hearing screening or further audiological assessment until they suspected an issue when Emma was 4 years old. This late identification and intervention is tracked as a “complex factor” in the present study. Emma was identified with a moderate to moderately-severe sensorineural hearing loss in the right ear and a moderate rising to mild sensorineural hearing loss in the left (PTA right = 51.7 dB HL; PTA left = 40.0 dB HL) and was fitted with hearing aids immediately. Following a fit to targets evaluation, the SII values were plotted on the Aided SII Normative Values Worksheet to conceptualize the audibility of the fitting relative to the normative data. Results indicated that the SII values for an average speech input (Right = 70%; Left = 75%; Figure 13) for Emma’s degree of hearing loss falls within the 95% confidence interval and therefore would be considered to have typical audibility. Therefore the clinician proceeded with using the functional outcome evaluation tools (i.e., LittlEARS, PEACH) with the knowledge that the hearing aid fitting was providing typical audibility for the child’s degree of hearing loss.
Figure 13.
Aided SII values for case example: Emma
Note: SII values (y-axis) for an average speech input are plotted for the right (O) and left (X) hearing aid fittings by Emma’s PTA (x-axis). Since the symbols fall within the 95% confidence intervals (dashed lines), it is concluded that Emma’s hearing aid fitting is providing a typical degree of audibility for her degree of hearing loss, in both ears.
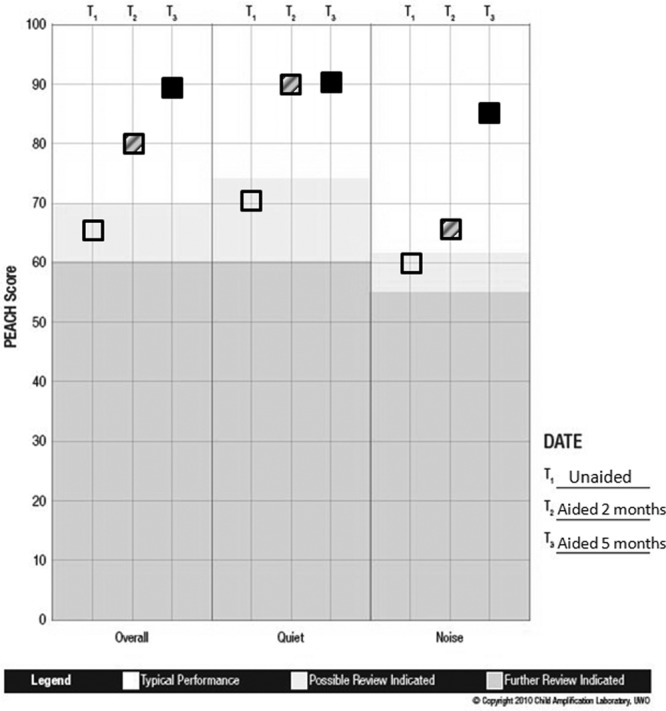
Emma is greater than 2 years of age and has normal developmental status. Therefore, prior to being fitted with hearing aids, Emma’s mother completed the PEACH. Scores ranged from 65%, 70%, to 60% for the overall, quiet, and noise subscales, respectively, for the unaided condition (Figure 14). After 2 months of experience with the hearing aids, Emma’s scores on the PEACH increased to 80%, 91%, and 65% for the same subscales. With 5 months of hearing aid experience, Emma’s scores improved to 88%, 91%, and 85% on the overall, quiet, and noise subscales, respectively, (Figure 13). An improvement in the noise score may have coincided with the introduction of a noise management program. This was prompted by the child’s descriptions of problematic listening while in the shopping center, which may not have been a topic of discussion had the PEACH not been administered.
Figure 14.
PEACH score sheet for case example: Emma
Note: The PEACH percentage scores (y-axis) are plotted within each subscale (x-axis) for this case example. Open squares indicate the unaided condition, hatched squares indicate 2 months of hearing aid use, and filled squares indicate 5 months of hearing aid use. Results indicate that prior to the use of hearing aids, Emma was demonstrating atypical auditory performance. As she gained experience with amplification, she demonstrated an improvement in auditory performance over time in all subscales.
This demonstrates that the PEACH is sensitive to auditory performance in the unaided and aided condition and shows progression in scores with more experience with hearing aids. In this case, a positive outcome with intervention was documented by systematically tracking the child’s auditory performance over time. Although this child was late-identified, which resulted in late intervention with hearing aids, initiating intervention that followed an evidence-based protocol improved the child’s auditory performance compared to when intervention was not provided.
Summary and Clinical Implications
Outcome evaluation is a key stage in the pediatric hearing aid fitting process. An evidence-based and clinically feasible guideline for systematically measuring the impact of hearing aid intervention in infants and young children has been an identified need in pediatric audiology (Moodie et al., 2011). A critical review of existing pediatric outcome evaluation tools revealed some caregiver-report functional outcome tools that have the characteristics to be included in a clinical guideline as well as be implemented clinically (Bagatto, Moodie, et al., 2011). With input from the Network of Pediatric Audiologists of Canada, the systematically chosen tools were included in the UWO PedAMP (Moodie et al., 2011). The first version of the UWO PedAMP includes outcome evaluation tools that aim to measure auditory-related outcomes in infants and young children who wear hearing aids, including subjective assessment of early auditory development (LittlEARS) and subjective ratings of auditory performance in daily life (PEACH). In addition, clinical process outcomes to assess the appropriateness of the hearing aid fitting are also included. Furthermore, their clinical implementation was supported by the data presented here along with administration guidelines and score sheets to help with interpretation. Overall, the work presented here will contribute to a better understanding of existing norms for the LittlEARS and the PEACH as well as provide a guideline for outcome evaluation for infants and children who wear hearing aids. Further work is required to characterize the performance of hearing-impaired children with varying clinical profiles. This is necessary for EHDI programs where hearing aids are a common intervention choice for families and outcome evaluation is an important stage of the hearing aid fitting process.
Acknowledgments
The authors gratefully acknowledge the input of Richard Seewald, Doreen Bartlett, and Martyn Hyde during the development stages of this work. The support of Christine Brown for her participation in data collection, Kelley Keene for her help with data organization, and Andrew Johnson for his assistance with data analysis are also appreciated.
List of Abbreviations
- AAA
American Academy of Audiology
- ANSD
Auditory neuropathy spectrum disorder
- ANSI
American National Standards Institute
- BCEHP
British Columbia Early Hearing Program
- CASLPO
College of Audiologists and Speech Language Pathologists of Ontario
- DSL
Desired Sensation Level
- EHDI
Early hearing detection and intervention
- JCIH
Joint Committee on Infant Hearing
- MCHAS
Modernizing Children’s Hearing Aid Services
- MPO
Maximum power output
- OIHP
Ontario Infant Hearing Program
- PCHI
Permanent childhood hearing impairment
- PEACH
Parents’ Evaluation of Aural/Oral Performance of Children
- PTA
Pure tone average
- REAR
Real-ear aided response
- RECD
Real-ear-to-coupler difference
- SII
Speech intelligibility index
- UWO PedAMP
University of Western Ontario Pediatric Audiological Monitoring Protocol
Appendix
(A) Location of Questionnaires
| Questionnaire/Outcome Tool | Location |
|---|---|
| Aided Speech Intelligibility Index (SII) Normative Values | www.dslio.com |
| Hearing Aid Fitting Summary | www.dslio.com |
| LittlEARS Auditory Questionnaire |
http://www.earfoundation.org.uk/shop/items/98 Otherlanguages direct from MED-EL. Tel: +44 (0) 1226 242 874 |
| PEACH Diary | http://www.nal.gov.au/outcome-measures_tab_peach.shtml |
| PEACH Rating Scale | http://www.outcomes.nal.gov.au/LOCHI%20assessments.html |
(B) LittlEARS Score Sheet
(C) PEACH Score Sheet
The first fitting of a device is usually tracked in the OIHP database. Data for those infants who received a cochlear implant following the use of a hearing instrument have not been uniformly tracked within the program. This may reduce the number of reported cochlear implant users in the OIHP relative to programs that track all children who receive cochlear implants regardless of referral path.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported with funding from the Canadian Institutes of Health Research (Marlene Bagatto: 200811CGV-204713-174463 and Sheila Moodie: 200710CGD-188113-171346) and the Ontario Research Fund, Early Researcher Award to Susan Scollie and the Early Learning and Child Development Branch, the Children’s Corporate Systems Branch of Ontario’s Ministry of Children and Youth Services in Canada, Siemens Hearing Instruments, Canada and The Masonic Foundation of Ontario, Help-2-Hear Project.
References
- American Academy of Audiology. (2003, October). Pediatric amplification protocol. Retrieved August 18, 2011 from http://www.audiology.org/resources/documentlibrary/Documents/pedamp.pdf
- American National Standards Institute. (1997). Methods for the calculation of the Speech Intelligibility Index (ANSI S3.5–1997). New York, NY: Acoustical Society of America [Google Scholar]
- Andresen E. M. (2000). Criteria for assessing the tools of disability outcomes research. Archives of Physical Medicine & Rehabilitation, 81(Suppl. 2), S15-S20 [DOI] [PubMed] [Google Scholar]
- Bagatto M. P., Brown C. L., Moodie S. T., Scollie S. D. (2011). External validation of the LittlEARS® auditory questionnaire with English-speaking families of Canadian children with normal hearing. International Journal of Pediatric Otorhinolaryngology, 75, 815-817 [DOI] [PubMed] [Google Scholar]
- Bagatto M. P., Moodie S. T., Seewald R.C., Bartlett D. J., Scollie S.D. (2011). A critical review of audiological outcome measures for infants and children. Trends in Amplification. 2011, 15:1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatto M. P., Scollie S. D., Hyde M. L., Seewald R. C. (2010). Protocol for the provision of amplification within the Ontario Infant Hearing Program. International Journal of Audiology, 49, S70-79 [DOI] [PubMed] [Google Scholar]
- Bagatto M. P., Scollie S. D., Seewald R. C., Moodie K. S., Hoover B. M. (2002). Real-ear-to-coupler difference predictions as a function of age for two coupling procedures. Journal of the American Academy of Audiology, 13, 407-415 [PubMed] [Google Scholar]
- Bass-Ringdahl S. M. (2010). The relationship of audibility and the development of canonical babbling in young children with hearing impairement. Journal of Deaf Studies and Deaf Education, 15, 287-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Columbia Early Hearing Program. (2006). Early Hearing Equipment Protocol Advisory Group. Retrieved August 18, 2011 from: http://www.phsa.ca/NR/rdonlyres/06D79FEB-D187-43E9-91E4-8C09959F38D8/40116/aHearingEquipmentProtocol.pdf
- Ching T. Y. C., Dillon H., Day J., Crowe K. (2007). The NAL study on longitudinal outcomes of hearing-impaired children: Interim findings on language of early- and later-identified children at 6 months after hearing aid fitting. In Seewald R. C., Bamford J. M. (Eds.), A sound foundation through early amplification: Proceedings of the Fourth International Conference (pp. 185-199). Stafa, Switzerland: Phonak AG [Google Scholar]
- Ching T. Y., Hill M. (2005a). The Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) Rating Scale. Chatswood, New South Wales, Australia: Australian Hearing; Retrieved August 18, 2011 from http://www.outcomes.nal.gov.au/LOCHI%20assessments.html [Google Scholar]
- Ching T. Y., Hill M. (2005b). The Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) Diary. Chatswood, New South Wales, Australia: Australian Hearing; Retrieved August 18, 2011 from http://www.nal.gov.au/outcome-measures_tab_peach.shtml [Google Scholar]
- Ching T., Hill M. (2007). The parents’ evaluation of aural/ oral performance of children (PEACH) scale: Normative data; Journal of the American Academy of Audiology, 18, 220-235 [DOI] [PubMed] [Google Scholar]
- Ching T., Hill M., Dillon H. (2008). Effect of variations in hearing-aid frequency response on real-life functional performance of children with severe or profound hearing loss. International Journal of Audiology, 47, 461-475 [DOI] [PubMed] [Google Scholar]
- College of Audiologists and Speech-Language Pathologists of Ontario. (2002). Preferred Practice Guideline for the Prescription of Hearing Aids to Children. Retrieved August 18, 2011 from http://www.caslpo.com/Portals/0/ppg/preshearingaidschild.pdf
- Coninx F., Weichbold V., Tsiakpini L., Autrique E., Bescond G., Chereches L., . . . Brachmaier J. (2009). Validation of the LittlEARS® Auditory Questionnaire in children with normal hearing. International Journal of Pediatric Otorhinolaryngology, 73, 1761-1768 [DOI] [PubMed] [Google Scholar]
- Golding M., Pearce W., Seymour J., Cooper A., Ching T., Dillon H. (2007). The relationship between Obligatory Cortical Auditory Evoked Potentials (CAEPs) and function measures in young infants. Journal of the American Academy of Audiology, 18, 117-125 [DOI] [PubMed] [Google Scholar]
- Harrison M. (2000). How do we know we’ve got it right? Observing performance with amplification. In Ed. R.C. Seewald, A sound foundation through early amplification: Proceedings of an International Conference (pp. 119-140). Stäfa, Switzerland: Phonak AG [Google Scholar]
- Joint Committee on Infant Hearing. (2007). Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics, 120, 898-921 [DOI] [PubMed] [Google Scholar]
- Kassirer J. P. (2010). Teaching clinical reasoning: Case-based and coached. Academic Medicine, 85, 1118-1124 [DOI] [PubMed] [Google Scholar]
- King A. M. (2010). The national protocol for paediatric amplification in Australia. International Journal of Audiology, 49, S64-69 [DOI] [PubMed] [Google Scholar]
- Kuehn-Inacker H., Weichbold V., Tsiakpini L., Coninx F., D’Haese P. (2003). LittlEARS Auditory Questionnaire: Parents questionnaire to assess auditory behaviour. Innsbruck, Austria: MED-EL [Google Scholar]
- Lee C. J. (2004). Horatio (Version 3.0) [Computer Software]. London, Ontario, Canada: Author; Retrieved August 18, 2011 from http://publish.uwo.ca/~cjlee/horatio/index.html [Google Scholar]
- Modernising Children’s Hearing Aid Services. (2005). Guidelines for the fitting, verification, and evaluation of digital signal processing hearing aids within a children’s hearing aid service. Retrieved August 18, 2011 from http://www.psych-sci.manchester.ac.uk/mchas/guidelines/fittingguidelines.doc
- Moeller M. P., Hoover B., Putman C., Arbataitis K., Bohnenkamp G., Peterson B., . . . Stelmachowicz P. G. (2007a). Vocalizations of infants with hearing loss compared with infants with normal hearing: Part I—Phonetic development. Ear and Hearing, 28, 605-627 [DOI] [PubMed] [Google Scholar]
- Moeller M. P., Hoover B., Putman C., Arbataitis K., Bohnenkamp G., Peterson B., . . . Stelmachowicz P. G. (2007b). Vocalizations of infants with hearing loss compared with infants with normal hearing: Part II—Transition to words. Ear and Hearing, 28, 628-642 [DOI] [PubMed] [Google Scholar]
- Moodie S. T. (2009). Clinician fit-to-DSL Targets: Preliminary data from a network study, Audiology Online. Retrieved August 18, 2011 from http://www.audiologyonline.com/ceus/livecoursedetails.asp?class_id=14417
- Moodie S. T. (2010, October 6). Engaging audiologists in research: The network of pediatric audiologists of Canada. Paper presented at the Canadian Academy of Audiology (CAA) Conference, Montreal, Quebec, Canada [Google Scholar]
- Moodie S. T., Bagatto M. P., Seewald R. C., Kothari A., Miller L., Scollie S. D. (2011). An integrated knowledge translation experience: Use of the Network of Pediatric Audiologists of Canada to facilitate the development of the University of Western Ontario Pediatric Audiological Monitoring Protocol (UWO PedAMP v1.0). Trends in Amplification, 15(1-2), 34-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollie S. D., Seewald R. C., Cornelisse L. C., Moodie S, Bagatto M, Laurnagaray D, . . . Pumford J. (2005). The Desired Sensation Level multistage input/output algorithm. Trends in Amplification, 9, 159-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sininger Y., Grimes A., Christensen E. (2010). Auditory development in early amplified children: Factors influencing auditory-based communication outcomes in children with hearing loss. Ear & Hearing, 31, 166-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiakpini L., Weichbold V., Kuehn-Inacker H., Coninx F, D’Haese P, Almadin S. (2004). LittlEARS Auditory Questionnaire. Innsbruck, Austria: MED-EL [Google Scholar]