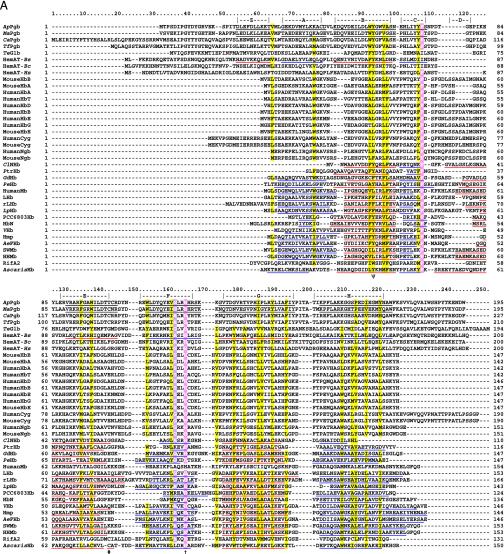
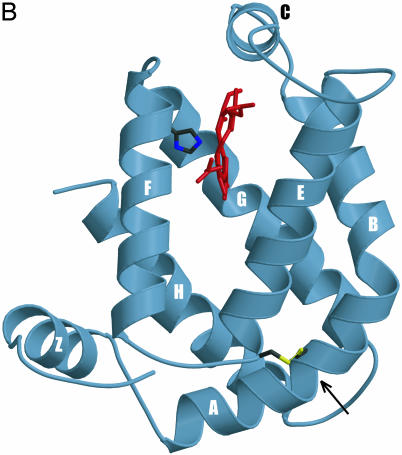
Fig. 1.
(A) Globin structural alignment with Pgbs. Secondary structure elements in globins with known 3D structures are underlined by color and labeled A through H whereas helices predicted by jpred are underlined without color. The pre-A helix is labeled Z, according to the HemAT-Bs structure. Positions at least 50% identical are indicated by bold characters, with the following notations: dagger (†), proximal F8 histidine; pound sign (#), E19 cysteine; psi (ψ), B10 Tyr. Color-coded amino acids are based on an 80% consensus sequence, and colored highlights are assigned to amino acid groups as follows: polar (p, KRHEDQNST) in red; turn-like (t, ACDEGHKNPQRST) in green; bulky hydrophobic (h, ACLIVMHYFW), and aliphatic (l, LIVM) in yellow; aromatic (a, FHWY) in white on pink background; small (s, ACDGNPSTV) in purple; and tiny (u, AGS) in white on purple background. The following sequences and structures were used (PDB IDs in parentheses): HumanMb (5MBN); HHMb (1WLA); SWMb (1MBO); AeFHb (1CQX); Hmp (1GVH); VHb (1VHB); rLHb (1D8U); LHb (2GDM); LpHb (1EBT); GdHb (1VRF); ClNHb (1KR7); PtrHb (1DLW); HbN (1IDR); PCC6803Hb (1MWB); PeHb (1H97); and AscarisHb (1ASH). (B) 3D homology model of ApPgb including the proximal histidine (dark green) and heme (red). The helices are labeled A through H with the disulfide bridge (yellow) indicated with an arrow. MouseHbA, -HbB, -HbE, -Cyg, and -Ngb stand for mouse Hb α, mouse Hb β, mouse Hb ε, mouse cytoglobin, and mouse neuroglobin. HumanHbA, -HbB, -HbG, -HbD, -HbE, -HbT, -Cyg, and -Ngb stand for human Hb α, human Hb β, human Hb γ, human Hb δ, human Hb ε, human Hb θ1, human cytoglobin, and human neuroglobin.