Abstract
The β-secretase, BACE1, generates β-amyloid (Aβ), a major hallmark of Alzheimer’s disease (AD) pathology. The elevation of BACE1 levels in brains of AD patients may play a role in initiating or propagating disease. BACE1 levels are increased under low energy or low oxygen conditions, which may occur in individuals with impaired circulation in the brain. We compared levels of BACE1 in the brains of aged, non-demented individuals with high or low levels of atherosclerosis in the circle of Willis, and found that while there is no change in BACE1, Aβ42 levels are elevated in the high atherosclerosis group.
Keywords: Aβ, Alzheimer’s disease, amyloid, atherosclerosis, BACE1, eIF2α
INTRODUCTION
BACE1, Beta-site Amyloid Precursor Protein Cleaving Enzyme 1, is the rate limiting enzyme in the generation of beta amyloid (Aβ) from Amyloid Precursor Protein (APP) [1, 2]. A β forms amyloid plaques and toxic diffusible oligomers in the brains of AD patients. BACE1 protein and activity are elevated in AD brains [3–5], suggesting that increased BACE1 could play a role in the development and/or progression of disease by increasing Aβ generation. BACE1 levels are upregulated during stresses associated with AD risk such as energy deprivation [4, 6], hypoxia and stroke [7, 8], and oxidative stress [9]. Under conditions of impaired glucose metabolism, phosphorylation of the alpha subunit of the eukaryotic initiation factor 2 (eIF2α) leads to increased BACE1 translation and Aβ generation, and eIF2α phosphorylation is elevated in AD brains [4].
Positron emission tomography has shown reduced glucose metabolism in brains of AD patients, and young, non-demented ApoE4 carriers, suggesting it plays a causative role in AD [10]. AD risk factors like stroke, traumatic brain injury and cardiovascular disease may result in impaired supply of glucose and oxygen to the brain, suggesting energy deficits in general could lead to AD. The watershed regions at the boundary zones of the three major cerebral arteries (anterior, middle and posterior) are especially susceptible to the effects of brain hypoperfusion [11]. Watershed microinfarcts are elevated 10-fold in the brains of AD patients compared to normal controls [12].
Prior studies have found a relation between cerebral atherosclerosis and AD pathology among persons with and without dementia [13–16]. We hypothesized that atherosclerosis leads to hypoperfusion of the brain and decreased glucose and oxygen supply. This could elevate eIF2α phosphorylation, leading to increased BACE1 activity and Aβ42 generation, resulting in increased risk of developing AD. This pathway could initiate in the watershed regions that are the first to suffer energy deficits during cerebral hypoperfusion and be present early in the disease, prior to the onset of dementia. We quantified BACE1, phospho-eIF2α and Aβ42 levels in watershed and non-watershed regions from non-demented, aged individuals with severe atherosclerosis of the circle of Willis compared to persons with little to no atherosclerosis. We found no increase in BACE1 or peIF2 α, however we found elevation of Aβ42 among those with atherosclerosis. Decreased Aβ clearance by the Aβ de-grading enzymes neprilysin and insulin degrading enzyme (IDE) [17] is not implicated, as levels of these proteins were not decreased in brains with atherosclerosis. We conclude that impaired cerebrovascular function elevates Aβ42 by an unknown mechanism, and BACE1 elevation occurs later in disease development.
MATERIALS AND METHODS
Immunoblotting
Postmortem samples of superior (watershed) and inferior (non-watershed) frontal cortex were obtained from nineteen non-demented aged participants based on a detailed clinical evaluation from the Rush Memory and Aging Project after Rush University IRB approval [18]. This included 10 with severe and 9 with little or no atherosclerosis based on semiquantitative analysis of the circle of Willis (Fig. 1A). Samples were homogenized in 1×PBS/1% TritonX-100 containing protease (Calbiochem) and phosphatase inhibitors (Pierce), and protein content quantified by BCA assay. 20µg of brain homogenate were separated on Invitrogen's 4–12% Bis-Tris NuPage Mini Gels, transferred to PVDF membrane, stained with Ponceau, scanned, then probed with anti-BACE1 antibody (3D5 1:1000) [3], anti-phospho-eIF2α (Epitomics, clone E90, 1:2000), anti-eIF2α (Cell Signaling, #9722, 1:2000), anti-neprilysin (Abcam ab79423, 1:1000) and anti-IDE (Abcam ab32216, 1:1000), followed by secondary HRP-conjugated antibody (Vector Laboratories 1:10,000). Blots were visualized using Luminata Crescendo (Millipore). Signals were captured and quantified using a Kodak Image Station 4000R digital imager. All signals were normalized to ponceau, except p-eIF2α was normalized to total eIF2α. To compare all 38 samples on one blot, gels were cut into horizontal strips and stacked so all samples for a given protein were transferred onto a single membrane. This eliminated variation in transfer, antibody incubation and ECL application that can occur between blots.
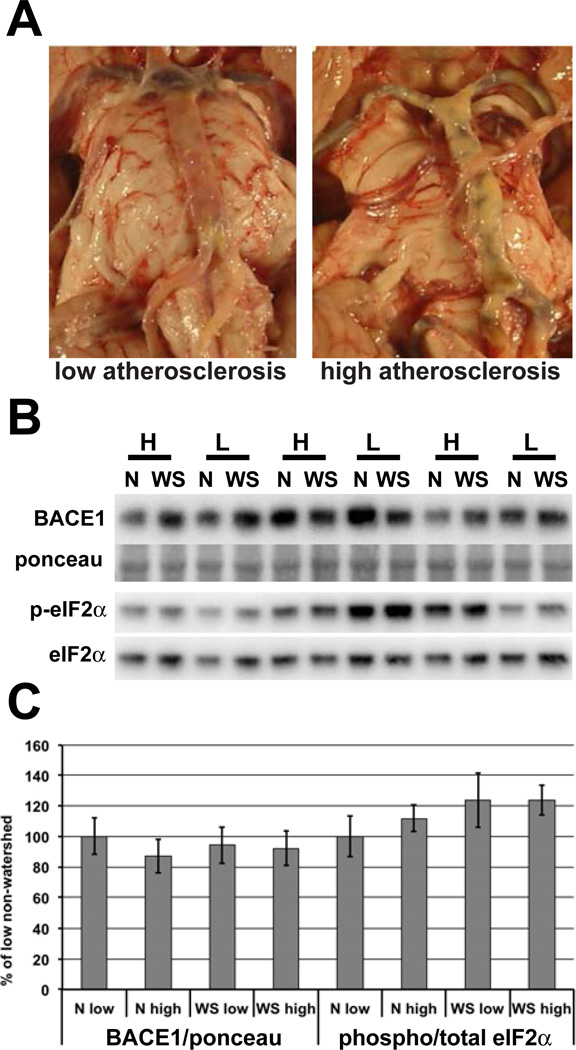
Fig. 1. BACE1 and phospho-eIF2α levels are not elevated in non-demented individuals with high cerebrovascular disease.
(A) Representative images of the circle of Willis from non-demented individuals categorized as having low or high levels of atherosclerosis. (B) Homogenates of human brain samples described in (Table 1) were analyzed by immunoblotting for BACE1 and phospho- and total eIF2α, as shown in representative blots. (C) Quantification of immunoblots shows no difference in BACE1 or ratio of phospho to total eIF2α in those with high levels of atherosclerosis compared to those with low atherosclerosis. The blot in (B) shows only 6 individuals in the interest of space. This is just a portion of the blot containing both watershed and non-watershed regions from all 19 individuals that was used in quantification. N=non-watershed, WS= watershed, H=high atherosclerosis, L=low atherosclerosis. p<0.05 *
Aβ42 Dot Blot and ELISA
10mg/ml brain homogenates were extracted in 5M guanidine hydrochloride (GuHCl) overnight on a nutator. For the WAKO Human β-Amyloid (1–42) Kit, GuHCl extracted samples were diluted 1:5 in PBS with protease inhibitors, then 1:100 into Standard Diluent, and ELISA was performed according to manufacturer's instructions. For dot blot, 1µl of GuHCl extracted sample (3.9µg of protein) was spotted in triplicate onto nitrocellulose membrane, dried one hour at 37°C, then stained with Ponceau. Duplicate blots were made and incubated in either 1:2500 anti-Aβ42 (Invitrogen, #700254) in 5% milk, or 5% milk only, followed by HRP-conjugated secondary antibody. Blots were developed with Luminata Crescendo (Millipore), and imaged simultaneously using the Kodak Image Station 4000R. The signal from the secondary-alone blot was subtracted from the Aβ42 signal to correct for any signal contributed by IgG cross reactivity, then all signals were normalized to Ponceau, and the triplicates averaged.
Statistical Analysis
Instat and Prism Graphpad were used to perform two tailed t-tests, linear regression, and to test for normal distributions. p<0.05 *
RESULTS
(Table 1) demonstrates that the high and low atherosclerosis groups were indistinguishable in terms of age, sex composition, years of eduation attained, and time since last administration of the mini-mental states exam (MMSE). They differed in the post-mortem interval (PMI) and score on last MMSE. The high atherosclerosis group had a shorter PMI and a lower last MMSE score. The distributions of all outcomes were consistent with a normal distribution.
Table 1. Summary of Research Subjects.
Frozen samples from non-watershed (inferior frontal cortex) and watershed (superior frontal cortex) regions of nineteen post-mortem brains were obtained from the Rush Memory and Aging Project. These individuals were all considered to be cognitively normal, and were categorized as having low atherosclerosis (n=9) or high atherosclerosis (n=10). The groups were indistinguishable in all measures, except for post-mortem interval and last MMSE score.
| Low Atherosclerosis | High Atherosclerosis | p value | |
|---|---|---|---|
| Age at death (yrs) | 86.1 ± 3.9 | 89.4 ± 6.6 | 0.2 |
| Sex | 22% male | 40% male | |
| Years of education | 14.4 ± 3 | 14.2 ± 2.4 | 0.8 |
| Last MMSE score (out of 30) | 29.1 ± 1.2 | 27.4 ± 1.6 | 0.02* |
| Days since last MMSE test | 341.5 ± 288.9 | 281.5 ± 282.6 | 0.7 |
| Sample size | n=9 | n=10 | |
| Postmortem interval | 6.8 ± 1.9 hrs | 5.0 ± 1.2 hrs | 0.02* |
| APOE4 hets | 4/9 | 1/10 |
Abbreviations: yrs, years; MMSE, mini-mental states exam; hets, heterozygotes for the APOE4 allele.
Analysis by immunoblotting showed no significant difference in BACE1 level in either watershed or non-watershed regions between the the low and high atherosclerosis groups (Fig. 1B, C). There was also no difference in BACE1 between the watershed and non-watershed regions in either group. The same was true for the ratio of phospho to total eIF2α, though there was a small trend for elevation in the watershed region, and for an increase in the non-watershed of the high atherosclerosis group compared to the low atherosclerosis group. These data indicate that neither eIF2α phosphorylation nor BACE1 levels in these regions are strongly affected by atherosclerosis or differ between watershed and non-watershed regions.
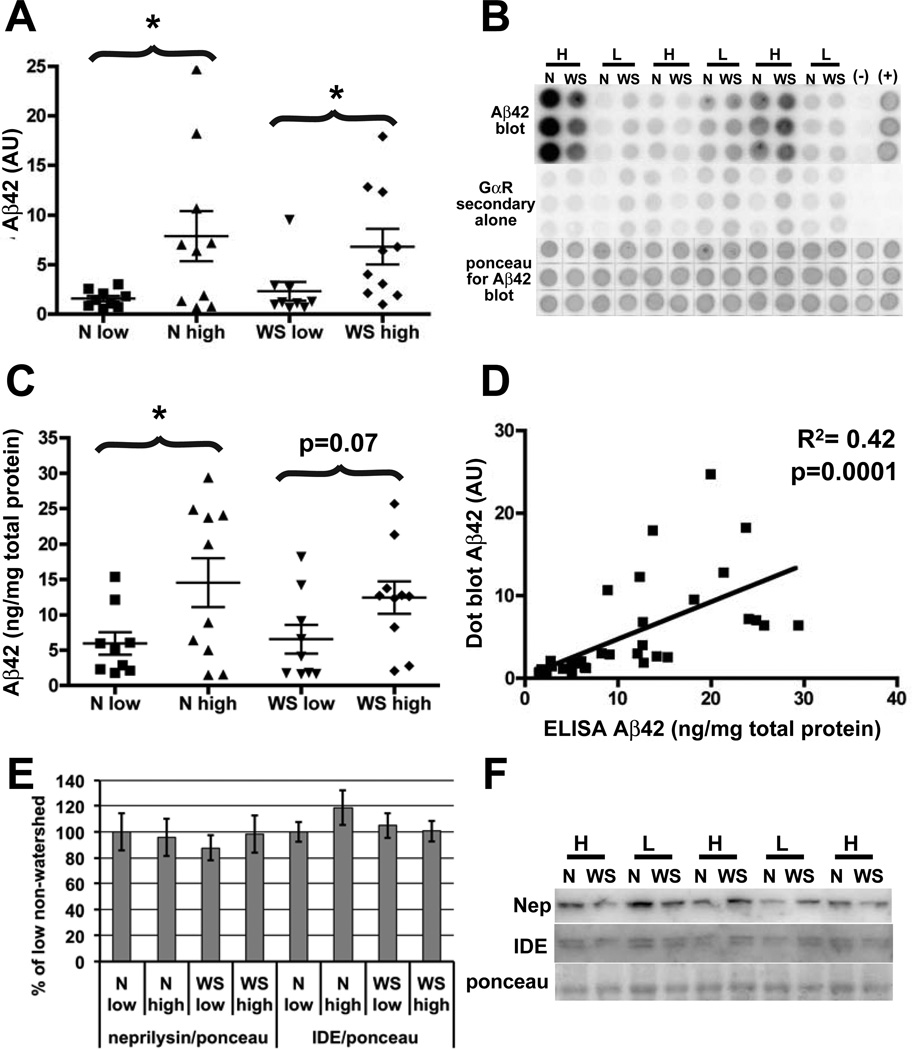
Aβ42-specific dot blots showed a significant increase in Aβ42 in both the watershed and non-watershed regions of the high atherosclerosis compared to the low atherosclerosis individuals (Fig. 2A, B). Aβ42-specific ELISA (Fig. 2C) showed elevation of Aβ42 in both brain regions in the high atherosclerosis group, though in the watershed region, this elevation did not reach statisitical significance (p=0.07). There was a significant correlation between the dot blot and ELISA results (R2=0.47, p=0.0001) validating the use of the Aβ42-specific dot blot for relative Aβ42 quantification (Fig. 2D). There was no difference in Aβ42 between watershed and non-watershed regions within the high and low atherosclerosis groups. There was no difference in levels of the Aβ degrading enzymes neprilyin and IDE in either watershed or non-watershed regions between the groups (Fig. 2E, F). Although BACE1 was not elevated on average in the high atherosclerosis group or in the watershed region as we had predicted, we performed linear regressions of BACE1 and Aβ42 to look for correlation between these proteins. There was no significant correlation between BACE1 and Aβ42 level in either watershed or nonwatershed regions, and this remained true whether high and low atherosclerosis samples were analyzed together or separately. This confirms that BACE1 elevation is not responsible for the observed Aβ42 increase. However, we cannot exclude the possibility that BACE1 levels would have become elevated had these individuals lived longer.
Fig. 2. Aβ42 levels are elevated in non-demented individuals with atherosclerosis, but neprilysin and insulin degrading enzyme levels are unchanged.
(A, B) Brain homogenates extracted in guanidine hydrochloride were analyzed by Aβ42-specific dot blot and quantified. (C, D). The accuracy of the Aβ42 dot blot was verified by subjecting the same samples to a commercial Aβ42-specific ELISA. The two methods yielded similar results. In addition, there is significant correlation between the level of Aβ42 measured by ELISA and dot blot (D). Combined, these data show an elevation in Aβ42 in the high atherosclerosis group in both watershed and non-watershed regions, and no difference between the regions in either low or high atherosclerosis groups. (E, F) Analysis by immunoblot and quantification shows that the increase in Aβ42 cannot be explained by a decrease in levels in either of the Aβ degrading enzymes, neprilysin or IDE as there is no difference between high and low atherosclerosis groups in either region. As in (Fig. 1), (B) and (F) show only 6 and 5 representative individuals respectively, in the interest of space, but these are just portions of the blots containing both watershed and non-watershed regions from all 19 individuals that were used in our analysis. N=non-watershed, WS= watershed, H=high atherosclerosis, L=low atherosclerosis.
DISCUSSION
We hypothesized that individuals with severe atherosclerosis would have elevated BACE1 due to chronic brain hypoperfusion. It was unexpected to find that we could already detect an increase in Aβ42 in the group with atherosclerosis but no change in BACE1. Increased Aβ42 in individuals with severe cerebral atherosclerosis is consistent with reports of increased Aβ in other conditions of impaired vascular function that could decrease energy and oxygen availability in the brain. After myocardial infarction, a longer duration of serum Aβ elevation corresponded to poorer outcome [19]. Non-demented patients with severe cardiovascular disease had elevated plaque counts compared to a control group [20].
In this study we focused on Aβ42 as this is considered to be more pathogenic than Aβ40 [21–23]. It would be of interest to determine whether Aβ40 increases in parallel with Aβ42 in individuals with cerebral atherosclerosis, and this information could shed light on the cause of Aβ42 elevation. We did not differentiate between soluble and insoluble Aβ42, or oligomers and monomers in this study. Since these individuals did not have detectable cognitive deficits, it is likely that levels of Aβ42, either soluble or insoluble, were below toxic thresholds for causing dementia. Data on amyloid load were only available for some of the individuals, but indicated that some of these cognitively intact individuals already had fibrillar Aβ deposited as plaques, in agreement with previous reports [24, 25].
While the groups of high and low atherosclerosis were equivalent in most measures, there were significant differences. The average PMI was significantly shorter for the high atherosclerosis group. This could have increased our ability to detect BACE1 and phospho-eIF2α as less degradation would have occurred, but there was no increase observed in these two proteins. We did observe an increase in Aβ42, but the Aβ peptide is relatively resistant to degradation. Other studies report increased Aβ or amyloid associated with circulatory problems such as infarct or cardiovascular disease, suggesting this is a reproducible finding [19, 20]. There were fewer APOE4 heterozygotes in those with atherosclerosis, which would lower the likelihood of amyloid, yet we still observed elevated Aβ42. The final MMSE score was lower in the high atherosclerosis group, suggesting that the increased Aβ, perhaps combined with cerebral hypoperfusion was already causing subtle cognitive impairment as seen with deposited amyloid in this group [26].
It is unclear how hypoxia might cause the elevation of Aβ in these high atherosclerosis individuals. It is possible that a small BACE1 elevation, undetectable by semiquantitative western blotting, is responsible. We did not detect any decrease in Aβ degrading enzymes neprilysin or IDE, but it is possible that Aβ42 is less efficiently cleared from the brain to the periphery via perivascular drainage pathways [27] in people with atherosclerosis. Our previous work demonstrated that Aβ42 could increase BACE1 [28], which in turn might cause increased Aβ generation, creating a positive feed forward mechanism in Alzheimer’s disease. We hypothesized that BACE1 elevation due to energy deprivation was the initiating step of this feed forward process [4–6]. However, the results presented here indicate that in individuals with cerebral atherosclerosis, Aβ elevation may be a first step in creating this feed forward scenario. This conclusion is supported by the observation that the atherosclerosis-associated increase in Aβ42 level occurred in the absence of a rise inBACE1 level, suggesting that elevation of Aβ42 precedes that of BACE1 during AD pathogenesis.
CONCLUSIONS
Our results suggest that atherosclerosis is an important pathogenic factor in preclinical AD, and underscore the potential benefit of controlling atherosclerosis for the prevention of AD. It will be important to understand how Aβis increased by cerebrovascular disease, and to determine if early Aβ42 elevation by similar or different mechanisms is common to other groups with increased risk of AD such as diabetics.
ACKNOWLEDGEMENTS
Human AD brain tissue samples were a gift from participants in Rush Hospital Memory and Aging Project (R01AG17917; Bennett, David A.; PI). This work was supported by the MetLife Foundation (RV) and NIH grants R01 AG030142 (RV) and F32AG033445 (KRS).
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
Author contributions: KRS designed research, collected data, analyzed data, and wrote paper. DAB designed research and edited paper. JAS collected data and edited paper. RV designed research and edited paper.
REFERENCES
- 1.Zheng H, Koo EH. Biology and pathophysiology of the amyloid precursor protein. Mol Neurodegener. 2011;6(1):27. doi: 10.1186/1750-1326-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O'Connor T, et al. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer's disease pathogenesis. J Neurosci. 2007;27(14):3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60(6):988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chami L, Checler F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and beta-amyloid production in Alzheimer's disease. Mol Neurodegener. 2012;7:52. doi: 10.1186/1750-1326-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velliquette RA, O'Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis. J Neurosci. 2005;25(47):10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103(49):18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004;1009(1–2):1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 9.Tong Y, Zhou W, Fung V, Christensen MA, Qing H, Sun X, et al. Oxidative stress potentiates BACE1 gene expression and Abeta generation. J Neural Transm. 2005;112(3):455–469. doi: 10.1007/s00702-004-0255-3. [DOI] [PubMed] [Google Scholar]
- 10.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miklossy J. Cerebral hypoperfusion induces cortical watershed microinfarcts which may further aggravate cognitive decline in Alzheimer's disease. Neurol Res. 2003;25(6):605–610. doi: 10.1179/016164103101202048. [DOI] [PubMed] [Google Scholar]
- 12.Suter OC, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke; a journal of cerebral circulation. 2002;33(8):1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 13.Roher AE, Tyas SL, Maarouf CL, Daugs ID, Kokjohn TA, Emmerling MR, et al. Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7(4):436–444. doi: 10.1016/j.jalz.2010.08.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta neuropathologica. 2007;113(1):13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 15.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol. 2003;23(11):2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 16.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain : a journal of neurology. 2012 Dec;135(Pt 12):3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol. 2008;18(2):240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zetterberg H, Mortberg E, Song L, Chang L, Provuncher GK, Patel PP, et al. Hypoxia due to cardiac arrest induces a time-dependent increase in serum amyloid beta levels in humans. PLoS One. 2011;6(12):e28263. doi: 10.1371/journal.pone.0028263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soneira CF, Scott TM. Severe cardiovascular disease and Alzheimer's disease: senile plaque formation in cortical areas. Clin Anat. 1996;9(2):118–127. doi: 10.1002/(SICI)1098-2353(1996)9:2<118::AID-CA4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101(5):1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer's disease: synapses gone cold. Mol Neurodegener. 2011;6(1):63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology. 1988;38(11):1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 26.Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol. 2012;72(4):599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell RD, Zlokovic BV. Neurovascular mechanisms and bloodbrain barrier disorder in Alzheimer's disease. Acta Neuro pathologica. 2009;118(1):103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadleir KR, Vassar R. Cdk5 protein inhibition and Abeta42 increase BACE1 protein level in primary neurons by a posttranscriptional mechanism: implications of CDK5 as a therapeutic target for Alzheimer disease. J Biol Chem. 2012;287(10):7224–7235. doi: 10.1074/jbc.M111.333914. [DOI] [PMC free article] [PubMed] [Google Scholar]