Abstract
HAMLET is the first member of a new family of tumoricidal protein-lipid complexes that kill cancer cells broadly, while sparing healthy, differentiated cells. Many and diverse tumor cell types are sensitive to the lethal effect, suggesting that HAMLET identifies and activates conserved death pathways in cancer cells. Here we investigated the molecular basis for the difference in sensitivity between cancer cells and healthy cells. Using a combination of small hairpin RNA inhibition, proteomic and metabolomic technology we identified the c-Myc oncogene as one essential determinant of HAMLET sensitivity. Increased c-Myc expression levels promoted the sensitivity to HAMLET and shRNA knockdown of c-Myc suppressed the lethal response, suggesting that oncogenic transformation with c-Myc creates a HAMLET-sensitive phenotype. Furthermore, the HAMLET sensitivity was modified by the glycolytic state of the tumor cells. Glucose deprivation sensitized tumor cells to HAMLET-induced cell death and in the shRNA screen Hexokinase 1, PFKFB1 and HIF1α modified HAMLET sensitivity. Hexokinase 1 was shown to bind HAMLET in a protein array containing approximately 8000 targets and Hexokinase activity decreased within 15 minutes of HAMLET treatment, prior to morphological signs of tumor cell death. In parallel, HAMLET triggered rapid metabolic paralysis in carcinoma cells. The glycolytic machinery was modified and glycolysis was shifted towards the pentose phosphate pathway. Tumor cells were also shown to contain large amounts of oleic acid and its derivatives already after 15 minutes. The results identify HAMLET as a novel anti-cancer agent that kills tumor cells by exploiting unifying features of cancer cells such as oncogene-addiction or the Warburg effect.
Keywords: HAMLET, metabolism, c-Myc, glycolysis
INTRODUCTION
Despite the molecular complexity of oncogenic transformation, cancer cells frequently suffer from ‘’oncogene addiction’’ and the disruption of a single oncogene can either reverse oncogenesis or be lethal (Weinstein and Joe 2008). The c-Myc oncogene is a classic example (Felsher and Bishop 1999) and is deregulated in at least 40% of all human cancers (Dang et al 2009). The broad transforming effect of c-Myc has been explained by its ability to bind to promoters of at least 30 % of all known genes (Dang et al 2009) and in transgenic mice, c-Myc overexpression combined with inhibition of apoptosis is sufficient to drive pancreatic cancer formation (Pelengaris et al 2002). More recently, inhibition of c-Myc was proposed to stop cancer growth and even allow tissue repair and reversion to a functional phenotype (Soucek et al 2008). Healthy cells, in contrast, retain a limited replicative potential, due to their susceptibility to programmed cell death, anti-growth signals and other molecular interactions that limit longevity and maintain normal tissue homeostasis (Hanahan and Weinberg 2000, Hanahan and Weinberg 2011).
Oncogene activity may also cause a shift in the glycolytic machinery (Hsu and Sabatini 2008) and cancer cells are highly dependent on glycolysis (Mathupala et al 1997). Glucose is first trapped inside cells by Hexokinases 1 and 2 (HK1/2) (Wilson 2003) and then further converted to pyruvate. In contrast to healthy cells, cancer cells drive pyruvate to lactate conversion even in the presence of oxygen, known as the Warburg effect (Warburg 1956). Recent studies have shown that oxidative phosphorylation is functional in most cancer cells (Vander Heiden et al 2009) and have proposed that the Warburg effect may reflect the expression of the pyruvate kinase isoform M2 (PKM2) (Christofk et al 2008), which catalyzes the dephosphorylation of phosphoenolpyruvate to pyruvate and is responsible for net ATP production within the glycolytic sequence. In contrast to healthy differentiated cells, embryonic cells and most cancer cells mainly express the M2 isoform (Mazurek et al 2005) and in some tumors, replacement of M2 by M1 reverses the Warburg effect and reduces tumorigenicity (Christofk et al 2008). In addition to PKM2, c-Myc plays a fundamental role in reprogramming the metabolism in certain tumor cells and has been shown to enhance aerobic glycolysis by directly activating genes such as HK2, PKM and LDHA (Dang et al 2009, Shim et al 1997). The transcription factor Hypoxia-inducible factor 1 (HIF1) also regulates glycolysis by enhancing the expression of glucose transporters, HKs and phosphofructokinases (PFKs) (Denko 2008).
HAMLET is a complex of partially unfolded α-lactalbumin and oleic acid, which preferentially kills cancer cells and immature cells (Svanborg et al 2003). The broad tumoricidal activity includes leukemia cells, carcinoma cells and glioma cells while healthy, differentiated cells survive HAMLET challenge (Fischer et al 2004a). HAMLET thus appears to target highly conserved survival mechanisms that are crucial for cancer cells. This selectivity has been confirmed in vivo in a human glioblastoma xenograft model and in a murine bladder cancer model (Fischer et al 2004b, Mossberg, 2010). In clinical studies of skin papillomas and bladder cancers (Fischer et al 2004b, Gustafsson et al 2004b, Mossberg et al 2007), HAMLET showed tumoricidal activity with limited side effects.
This study investigated the molecular basis of HAMLET sensitivity in cancer cells, using screening techniques to detect features of transcription, protein interaction and metabolism that alter survival after HAMLET exposure. We show that the sensitivity to HAMLET is dependent on c-Myc and glycolysis and identified the glycolytic enzyme HK1 as a potential direct target of HAMLET. The results also indicated that HAMLET alters cancer cell metabolism and that it may disrupt glycolysis which is altered in cancer cells due to the Warburg effect.
RESULTS
An shRNA screen identifies c-Myc and glycolytic-related proteins as modifiers of HAMLET sensitivity
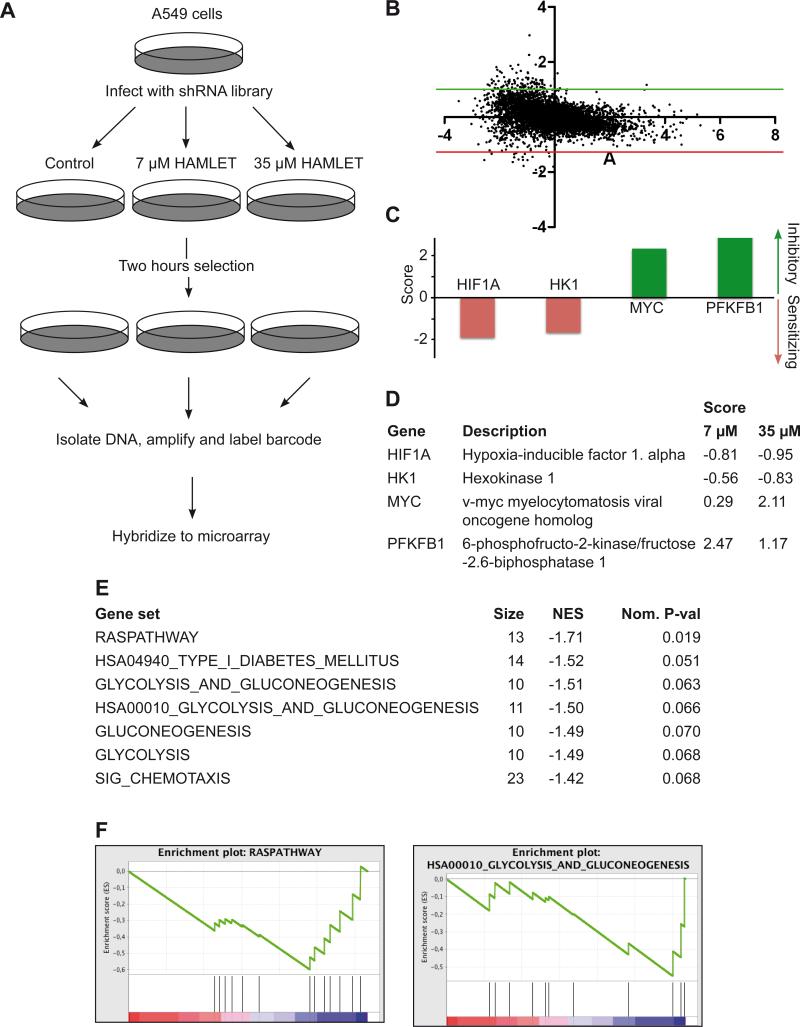
To get an unbiased overview of genes important for HAMLET sensitivity we utilized the RNAi barcode screening technology (Brummelkamp and Bernards 2003, Silva et al 2005, Silva et al 2008). Barcode screens use DNA microarrays to follow the relative abundance of each shRNA vector in a large population of cells infected with a shRNA vector library (Figure 1A). Using retroviral infection, we introduced a collection of 4603 shRNAs designed to target 1650 cancer-related human genes for suppression by RNA interference into A549 lung carcinoma cells, which are known to be HAMLET-sensitive. The infected cells were split into three pools, one of which was left untreated and was used as a reference while the other two were exposed to low (7 μM) and high (35 μM) concentrations of HAMLET. After 2 hours of HAMLET treatment, we recovered shRNA cassettes by PCR amplification and analyzed their relative abundance by hybridization to DNA microarrays as described (Zender et al 2008).
Figure 1. A shRNA barcode screen identifies c-Myc and metabolic proteins as determinants of HAMLET sensitivity.
A Workflow of the shRNA screen. A549 cells were infected with a library of barcoded shRNAs targeting 1650 cancer-related genes. After 2 hours of HAMLET exposure, barcodes were recovered, amplified and labeled with Cy3/Cy5 and the relative abundance of each shRNA was measured by microarrays. B A small number of hairpins were enriched, as seen in the MA-plot of shRNAs in control samples (Cy3) vs. HAMLET (35 μM) samples (Cy5). The fold change is shown on the x-axis (log2 (Cy5/Cy3) and the XXX is shown on the y-axis (log2 (√intensity Cy3 x intensity Cy5)). C-D shRNAs targeting HK1 and the metabolism-regulating genes PFKFB1, HIF1A and c-Myc significantly modify HAMLET sensitivity. The diagram shows the highest scores observed for each gene. E-F The gene list containing all positive hits from the shRNA screen was subjected to Gene Set Enrichment Analysis using the canonical pathway collection to identify common pathways targeted by the positive shRNAs. The Ras pathway, together with glycolysis-related pathways, had the highest normalized enrichment scores (NES).
A small number of shRNA vectors were enriched or depleted in the population treated with HAMLET; 43 inhibitory and 44 sensitizing shRNAs showed an absolute MaxMean score > 0.5 in both high and low dose treatment groups or an absolute score >2 in any group (Figure 1B and Table S1). The identified genes included oncogenes like c-Myc and glycolysis-related proteins. shRNAs against HK1 and Hypoxia-inducible factor 1 alpha (HIF1A) sensitized the cells to HAMLET (Figure 1C and 1D), suggesting that increased HK1 and HIF1A expression drives a phenotype more resistant to HAMLET. In contrast, shRNA targeting 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB1) and c-Myc rescued the cells (Figure 1C and 1D). Biological pathways enriched for by HAMLET were identified by Gene Set Enrichment Analysis (GSEA) using the canonical pathways gene set collection. The GSEA algorithm tests for the enrichment of a set of items within a larger ranked list of such items. The identified pathways confirmed the importance of glycolysis, as four glycolysis-related pathways significantly modified HAMLET sensitivity (Figure 1E and 1F). In addition, the Ras pathway, which governs c-Myc expression, was identified as a determinant of HAMLET sensitivity (Figure 1F).
The shRNA screen suggested that oncogenic transformation by c-Myc sensitizes cells to HAMLET-induced cell death and that the glycolytic machinery influences the HAMLET sensitivity of cancer cells.
The c-Myc oncogene determines the tumoricidal effect of HAMLET
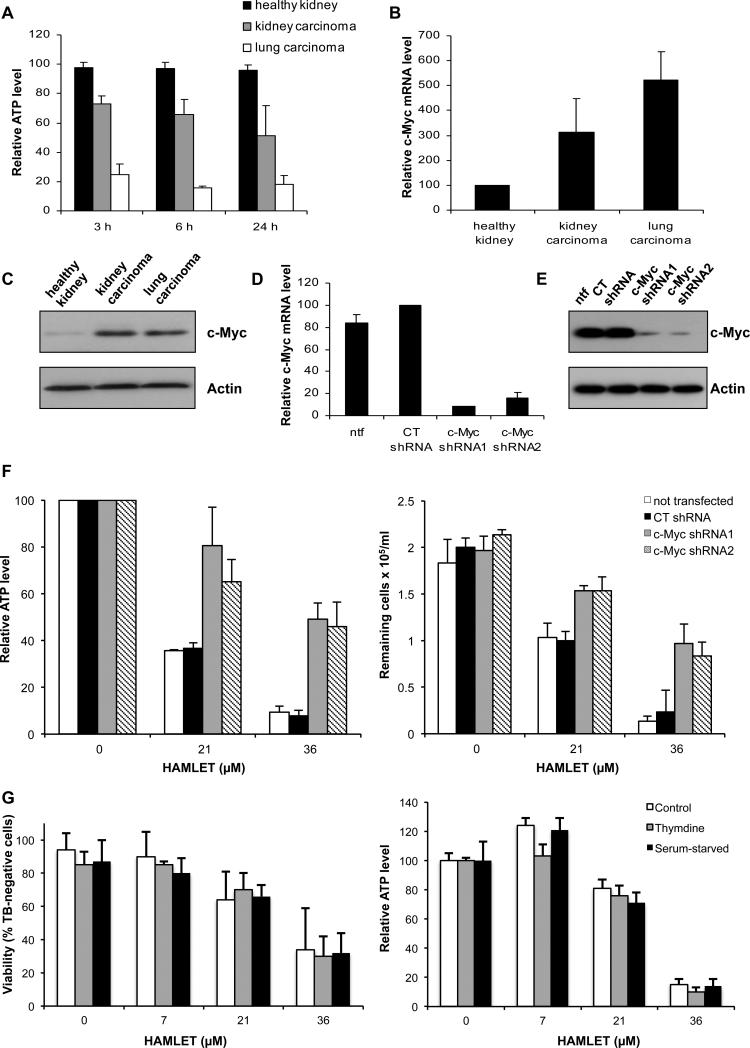
We subsequently examined if the HAMLET sensitivity reflects c-Myc expression levels. Human lung carcinoma cells (A549), kidney carcinoma cells (A498) and semi-differentiated healthy kidney cells (HRPTEC) were exposed to HAMLET (21 μM) and ATP levels were monitored (Figure 2A). A rapid reduction in ATP levels was observed in the lung and kidney carcinoma cells but healthy cells remained unaffected after 3, 6 and 24 hours. At higher HAMLET concentrations, a partial response was also seen also in those cells, however (Figure S1A). The difference in c-Myc expression reflected the difference in HAMLET sensitivity, as lung and kidney carcinoma cells show higher expression of c-Myc than healthy cells (Figure 2B and 2C).
Figure 2. c-Myc modifies sensitivity to HAMLET-induced cell death.
A Healthy kidney cells (HRPTEC), kidney carcinoma cells (A498) and lung carcinoma cells (A549) were treated with HAMLET (21 μM) and ATP levels were measured after 3, 6 and 24 hours. HAMLET caused a reduction in ATP levels in the carcinoma cells but not in the healthy cells. ATP levels are given in % of untreated cells at the same time point. Means of 3 experiments + SEMs. B qPCR quantification of c-Myc mRNA levels. Relative c-Myc mRNA levels are shown (c-Myc/GAPDH, in % of levels in healthy cells). Means of 2 experiments + SEMs. C c-Myc Western blot with Actin as a loading control. D, E Inhibition of c-Myc expression by transfection of A549 cells with c-Myc shRNAs (72 hours) was confirmed by qPCR (D) and c-Myc Western blot with Actin as loading control (E). Relative c-Myc mRNA levels are shown (c-Myc/GAPDH, in % of levels in CT shRNA-transfected cells). Means of 2 experiments + SEMs. ntf = non-transfected. F c-Myc shRNA-transfected A549 cells (72 hours) become more resistant to the lethal effect of HAMLET. The cytotoxic effect of HAMLET was quantified after 3 hours as a reduction in ATP levels or in the number of cells, compared to non-transfected cells (ntf) and cells transfected with CT shRNA. ATP levels in % of levels in respective untreated cells. Means of 2-3 experiments ± SEMs (ATP) or means of triplicates in 1 experiment + SDs (Cell survival). G A549 cells were cell cycle-arrested by serum starvation (48 hours) or double thymidine block and treated with HAMLET. Cancer cell viability was assessed after 3 hours after using Trypan blue exclusion or ATP measurements. Means ± SEMs of 3 experiments.
To address if c-Myc activity influences HAMLET-induced cell death, A549 lung carcinoma cells were transiently transfected with inhibitory c-Myc shRNAs or irrelevant shRNA and reduction in c-Myc expression was confirmed after 72 hours (Figure 2D and 2E). Cells with reduced c-Myc expression became more resistant to the lethal effects of HAMLET (21, 36 μM, 3 hours), while cells transfected with irrelevant shRNA retained their sensitivity, with an LC50 similar to untransfected cells (Figure 2F). The results suggest that the level of c-Myc expression is a direct determinant of HAMLET susceptibility in cancer cells.
As knockdown of c-Myc causes growth arrest in many cell lines (Watson et al 1991), we deliberately induced growth arrest and tested the effect on HAMLET sensitivity. A549 lung carcinoma cells were subjected to serum withdrawal or double-thymidine block and growth arrest was confirmed by flow cytometry after propidium iodide staining (Figure S1B). Growth arrest did not significantly alter the HAMLET-sensitivity (Figure 2G), indicating that the effect of c-Myc on HAMLET sensitivity is independent of the growth arrest induced by c-Myc knock down.
Glucose deprivation and inhibitors of glycolysis enhance HAMLET-induced cell death
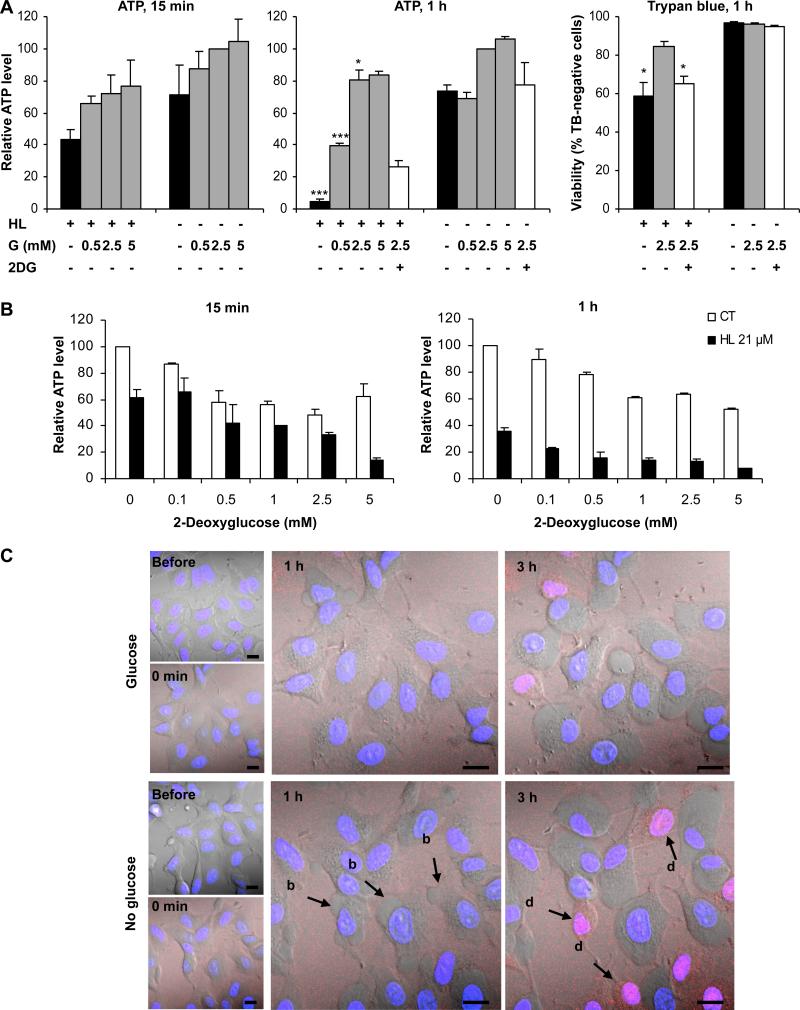
The shRNA screen and the GSEA analysis identified glycolysis–related proteins as determinants of HAMLET sensitivity. To directly address if glycolysis modifies the sensitivity to HAMLET, A549 lung carcinoma cells were deprived of glucose for 30 minutes, HAMLET was added (21 and 36 μM) and cell death was quantified (15 minutes, 1 and 3 hours). Glucose deprivation sensitized the cells to HAMLET, causing a dramatic reduction in ATP levels and Trypan blue exclusion as well as an increase in LDH release and this effect was reversed by the addition of glucose (Figure 3A and Figure S2A-C). In addition, the glucose analogue 2-deoxyglucose (2DG), which inhibits glycolysis, enhanced HAMLET toxicity in a dose-dependent manner (Figure 3B and Figure S2D, E).
Figure 3. Glucose deprivation and glycolysis inhibition enhances HAMLET-induced cell death.
Glucose (G) deprivation and the glycolytic inhibitor 2-deoxyglucose (2DG) enhance the lethal effect of HAMLET while glucose (0.5, 2.5, 5.0 mM) rescues the cells in a dose-dependent manner. The cytotoxic effect of HAMLET was quantified by measuring ATP levels or by Trypan blue (TB) exclusion and changes in morphology were recorded by confocal microscopy. A Glucose deprivation or 2DG (2.5 mM) increased the cytotoxic effect of HAMLET (HL, 21 μM) in A549 cells. ATP levels are given in % of levels in untreated cells in 2.5 mM glucose. Means of 2-4 experiments + SEMs (1 hour) * for p ≤ 0.05, *** for p ≤ 0.001 (vs. HL, 5 mM glucose for ATP and vs. HL, 2.5 mM glucose for TB). B Glycolysis inhibition by 2DG enhanced the lethal effect of HAMLET in glucose-deprived A549 cells in a dose-dependent manner. ATP levels in % of the levels in untreated cells. Means of 2 experiments + SEMs. C Glucose deprivation enhanced blebbing (indicated by arrow, b) and increased the number of rounded, dead cells that were strongly stained with HAMLET (indicated by arrow, d). A549 cells were deprived of glucose, treated with Alexa Flour 568-labeled HAMLET (21 μM, red) and changes in morphology were recorded by live-cell confocal microscopy over 3 hours. Nuclei are visualized with Hoechst staining (blue). Scale bars: 10 μm.
To further examine the effects of glucose on the cellular response to HAMLET, Alexa Fluor 568-labelled HAMLET was added to cancer cells stained with Hoechst and examined by real time confocal imaging. Glucose-deprived cells showed an increase in blebbing after 1 hour compared to glucose-fed cells and after 3 hours, more of the glucose-deprived cells had changed morphology and showed strong HAMLET staining (Figure 3C). Furthermore, 3-bromopyruvate, which inhibits glycolysis as well as mitochondrial ATP production (Ihrlund et al 2008), significantly increased HAMLET-induced cell death (Figure S2F). Together, these results show that cancer cells are sensitized to the lethal effects of HAMLET when glycolysis is inhibited.
HAMLET binds to Hexokinase
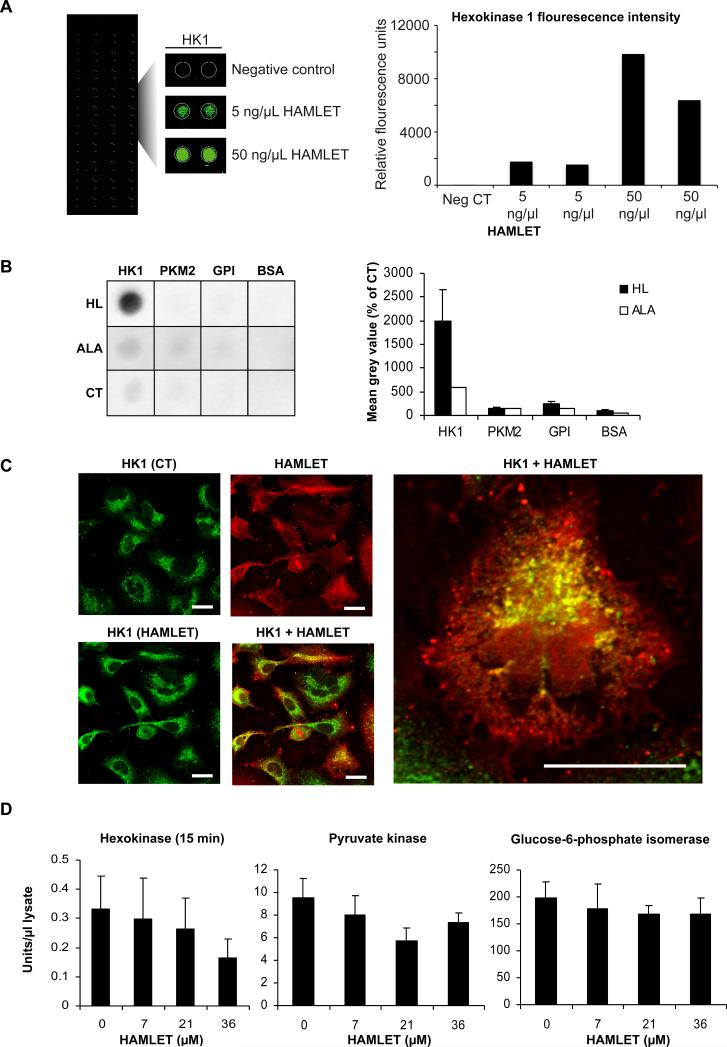
Potential binding partners of HAMLET in the glycolytic pathway were identified using a high content functional protein array on which 8000 human recombinant proteins were spotted (Figure 4A). Using stringent statistical filtering, 87 significant hits were identified including nucleotide-binding proteins, protein kinases and GTP-binding proteins. The glycolytic enzyme HK1 with a Z-score of 5.09 (Figure 4A) was one of the top scoring hits. In addition, several glycolytic enzymes showed increasing signal intensity with rising HAMLET concentrations but with lower Z-scores thus not meeting the stringent criteria for a positive hit in this screen (Z-score >3 and replicate spot and inter-array CV < 50%). These enzymes included PFKFB2 and 4 (Z-score 0.3 and 0.9), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Z-score 0.5) and PKM2 (Z-score 0.1). To confirm the binding of HAMLET to the glycolytic enzymes, PVDF membranes were spotted with recombinant His-tagged human HK1 and PKM2, rabbit GPI (93 % identical with human) and bovine serum albumin (BSA) as a negative control. The membranes were sequentially incubated with HAMLET or α-lactalbumin, goat anti-α-lactalbumin antibody and rabbit anti-goat HRP-conjugated antibody. HAMLET bound strongly to human HK1 but not to human PKM2, rabbit GPI or BSA (Figure 4B). This strong interaction was not seen with α-lactalbumin, suggesting that the HAMLET complex has significantly higher affinity for HK1 than the native, fatty acid-free protein.
Figure 4. HAMLET binds to HK 1.
A In a protein array containing 8000 proteins, HAMLET bound to HK1 in a dose-dependent manner (5 or 50 ng/μl) but not to PKM2 or other glycolytic enzymes. B In a far-Western dot blot, HAMLET bound strongly to human HK1 but not to the other three proteins. Recombinant human His-tagged HK1 and PKM2, rabbit muscle GPI and BSA were spotted onto PVDF membranes and incubated with HAMLET (HL) or α-lactalbumin (ALA) (both 0.2 mg/ml) or buffer as control. Bound HAMLET or α-lactalbumin was detected using goat anti-α-lactalbumin and HRP-conjugated rabbit anti-goat antibodies and quantified using ImageJ. Means of 4 (HK1 and PKM2) or 7 (GPI, BSA) experiments + SEMs for HAMLET. Results from 1 experiment for α-lactalbumin. C HAMLET colocalized with HK1 especially in the perinuclear region. Lung carcinoma cells were treated with Alexa Fluor 568-labelled HAMLET (HL, red) for 15 minutes and stained with anti-HK1 and Alexa Fluor 488-labelled antibodies (green). Scale bars: 20 μM. D HAMLET reduced HK activity but not Pyruvate Kinase or Glucose-6-phosphate isomerase activity, in a dose-dependent manner. Lung carcinoma cells were treated with different HAMLET concentrations for 15 minutes, lysed and activity in the lysates was quantified by a coupled spectrophotometric assay. Means of 3 experiments + SEMs.
Confocal microscopy was used to visualize HAMLET's interaction with HK1 in cancer cells. A549 lung carcinoma cells were treated with Alexa Fluor 568-labelled HAMLET (15 minutes, 21 μM), fixed and stained with anti-HK1 antibodies (Figure 5C). Cells stained with anti-PKM2 antibodies were used as controls (Figure S3). Untreated cells showed granular HK1 staining with the highest intensity in the perinuclear region (Figure 5C). In HAMLET-treated cells, strong colocalization between HAMLET and HK1 was observed especially in perinuclear, rounded aggregates. In contrast, only weak colocalization of PKM2 with HAMLET was detected (Figure S3).
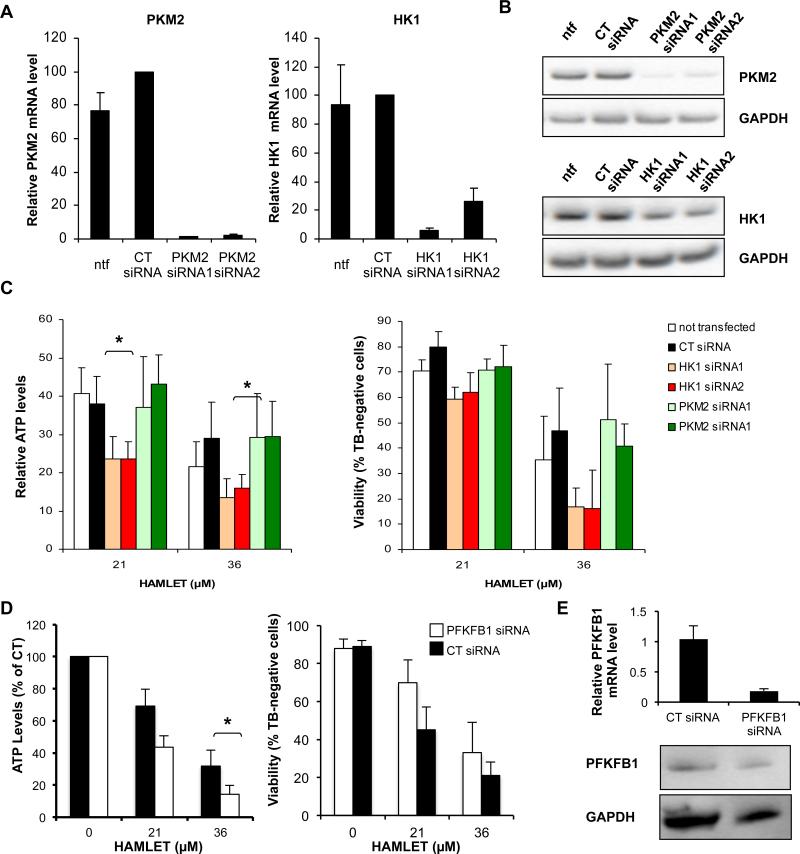
Figure 5. Knockdown of glycolysis-related proteins alters HAMLET sensitivity.
A, B A549 cells were transfected with control siRNAs or siRNAs targeting HK1 or PKM2. After 2 days knockdown was confirmed by qPCR (A) and Western blot with GAPDH as loading control (B). Relative mRNA levels are shown (PKM2/PPIA and HK1/PPIA in % of levels in CT siRNA-transfected cells). Means of 3-5 experiments + SEMs. C HK1 siRNAs enhanced the cytotoxic effect of HAMLET in A549 cells. A549 cells were transfected with siRNAs targeting HK1, control siRNA or siRNAs targeting PKM2 and, after 2 days, treated with HAMLET (3 hours). ATP levels and viability in % of levels in respective untreated cells. Means of 3-5 experiments + SEMs. * for p < 0.05. D, E PFKFB1 siRNA decreased the cytotoxic effect of HAMLET in A549 cells. A549 cells were transfected with PFKFB1 siRNA or control siRNA for 48 hours and treated with HAMLET. D ATP levels in % of levels in respective untreated cells. Means of 3 experiments + SEMs. * for p < 0.05 E Knockdown of PFKFB1 was confirmed by qPCR and Western blot.
HAMLET reduces HK activity in cancer cells
To determine if HAMLET disturbs glycolysis in cancer cells, HK, PK and GPI activity was measured in detergent-free lysates from HAMLET-treated A549 lung carcinoma cells (7, 21 and 36 μM) by coupled spectrophotometric assays. HK activity showed a dose-dependent trend towards a decrease after 15 minutes of HAMLET treatment (Figure 4D) but GPI and PK activities were not significantly altered. At this early time point, there were no changes in cell morphology or in cellular HK1 levels (Figure S4A and S4B). ATP levels decreased in a dose-dependent manner already at 15 minutes but lactate production was only reduced after 3 hours (Figure S4C). The results suggest that HAMLET may reduce HK activity in lung carcinoma cells.
Knockdown of HK1 or PFKFB1 modulates HAMLET sensitivity
To verify the shRNA screen and to father examine the role of glycolysis-related proteins in HAMLET-induced cell death, A549 lung carcinoma cells were transiently transfected with siRNAs targeting HK1, PFKFB1 and PKM2. Knockdown was confirmed by RT-PCR and Western blots (Figure 5A, B and E) and the sensitivity to HAMLET was quantified by measuring ATP levels and Trypan blue exclusion (Figure 5C, D). HK1 siRNA-transfected cells showed a more pronounced loss of ATP in response to HAMLET (21 and 36 μM, p < 0.05) than the untransfected cells or cells transfected with control siRNA. In contrast, PKM2 siRNA did not change the sensitivity to HAMLET (Figure 5C). Similar results were observed with Trypan blue exclusion Furthermore, knockdown of PFKFB1, which conferred resistance in the shRNA-screen, decreased the sensitivity to HAMLET (Figure 5D). These results confirm that knockdown of HK1 and PFKFB1 increase and reduce HAMLET sensitivity, respectively.
HAMLET rapidly disrupts cancer cell metabolism
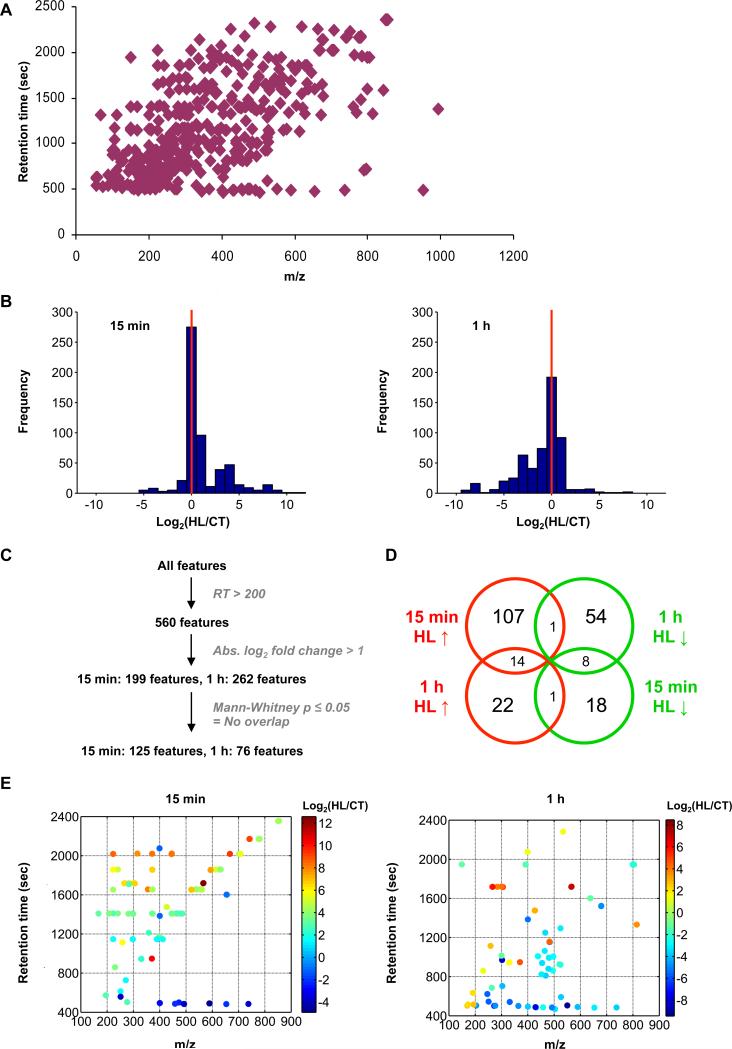
To assess if HAMLET changes the metabolic state of target cells, we compared the metabolomic profile of HAMLET-treated lung carcinoma cells (36 μM, 15 minutes and 1 hour) to untreated cells (Figure 6 and Figure S5). Three different untargeted metabolite profiling methods were used to study the possible metabolic effects of HAMLET. Reverse phase liquid chromatography coupled to electrospray ionization mass spectrometry (LC/MS) was used to compare hydrophobic metabolites (e.g. lipids), hydrophilic interaction chromatography (HILIC), LC-MS to study a wide range of polar metabolites, and gas chromatography mass spectrometry (GC/MS) to profile core metabolites (Baran et al 2009). By reverse phase LC-MS profiling 560 features were detected (including different adducts, isotopes and in source degradation products of the same metabolite) defined by m/z value and LC retention time (For the combination of m/z value and retention time, see Figure 6A). The analysis revealed that 125 of the 560 features were significantly altered after 15 minutes of HAMLET treatment (107 increased, 18 reduced). After 1 hour, the majority of 76 altered features were instead reduced (22 increased, 54 reduced) (Figure 6D). As expected from the uptake of HAMLET, the features with largest signal intensity in the HAMLET-treated cells at both time points as well as several features with similar retention time were putatively identified as different ionization and isotope variants of oleic acid, the lipid component of HAMLET, based on exact mass and LC retention time.
Figure 6. HAMLET disrupts cancer cell metabolism.
A-E Changes in metabolites in HAMLET-treated (15 minutes and 1 hour) A549 cells were analyzed by LC-MS reverse phase chromatography. A In total, 520 features, defined by retention time and m/z value, were detected. B Frequency distribution of log2-transformed fold changes in signal intensity between HAMLET-treated and control cells. After a rapid increase in average signal intensity after 15 minutes, a decrease in average signal intensity was observed in response to HAMLET. C Features showing an abs. log2 fold change ≥ 1 and p values ≤ 0.05 were considered significantly altered. D Using this definition, 125 features were found to be altered in the cells treated with HAMLET for 15 minutes and 76 features in the cells treated for 1 hour compared to the respective control cells. 24 of these features were significantly changed at both time points. E Plots of the significantly altered features with log2-transformed fold change indicated by colors.
Untargeted profiling using HILIC LC-MS was consistent with the reverse phase analysis, showing significant metabolic alterations after HAMLET treatment. Of the 1120 features that were detected, 103 were found to change after 15 minutes (28 were increased, 75 were reduced in HAMLET treated cells) and 114 after 1 hour of HAMLET treatment (64 were increased, 50 were reduced in HAMLET-treated cells). Several of these metabolites were identified based on comparison of exact mass and retention time with analytical standards. S-adenosyl-L-homocysteine, creatine, phosphocreatine, taurine and glutathione disulfide all decreased in the HAMLET-treated cells, possibly reflecting a disruption in amino acid metabolism and oxidative stress. The identity of creatine and glutathione disulfide was also confirmed by subsequent MS/MS analysis. The results indicate that HAMLET rapidly alters cancer cell metabolism.
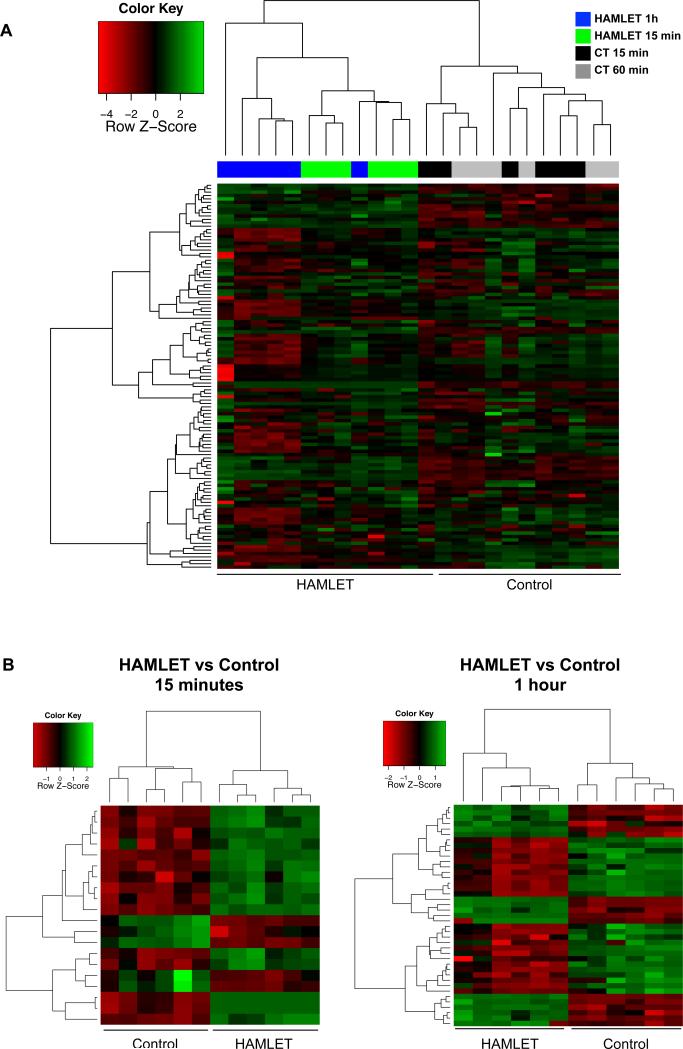
GC-MS profiling of cellular metabolites was subsequently performed in A549 lung carcinoma cells treated with HAMLET for 15 and 60 minutes. By hierarchical clustering, three separate clusters were observed, the first containing the control samples (15 and 60 minutes), the second the HAMLET-treated samples obtained after 15 and the third the 60 minutes samples, respectively (Figure 7A, Table S2). Overall metabolite concentrations were lower in the HAMLET-60 samples compared to controls, confirming the general shut down of metabolism identified in the untargeted profiling. To derive significantly altered metabolites, a linear model was fitted to the data and metabolites with an absolute log2-fold change of 1 and FDR-adjusted p-values < 0.05 were considered significant. Twenty metabolites were significantly altered after 15 minutes of HAMLET treatment (Figure 7B). As expected from the known uptake of HAMLET, oleic acid and the oleic acid derivative elaidic acid showed the most marked increase (120 fold, adjusted p-value = 4 × 10−6 and 84 and 8.1 × 10−6, respectively). In addition, a number of metabolites relating to the pentose phosphate pathway were more abundant after 15 minutes, including gluconic acid (3.3 fold), gluconate-lactone (2.6 fold), glyceric acid (2 fold) and pyruvate (1.7 fold) indicating that HAMLETs binding to HK might divert the metabolic flux towards this pathway. Among the metabolites that were significantly reduced were taurine, glycine, the citric acid cycle intermediate aconitate and the polyamines putrescine and spermidine After 1 hour, 41 metabolites were identified as significantly altered (Figure 7B). Oleic acid (log2 fold change of 5.1 and p=2 × 10−10) showed the second most marked increase together with derivatives including elaidic acid and palmitoleic acid. Pyruvate and several other intermediates in the citric acid cycle, including succinate, aconitate, fumarate and malate, were less abundant after HAMLET treatment, confirming HAMLETs inhibitory effect on glycolysis. Additionally, a number of key metabolites in arginine and proline metabolism were reduced, including aspartate, glutamate and spermidine. Finally, confirming the untargeted metabolomic analysis, HAMLET caused a depletion of metabolites relating to glutathione metabolism. Together, these results show that HAMLET rapidly causes a massive alteration of cancer cell metabolism which may in part result from a disruption of glycolysis.
Figure 7. Hierarchical clustering based on the results from the GC-MS analysis separates HAMLET and control samples.
A, B 113 metabolites were analyzed using GC-MS 15 minutes and 1 hour after HAMLET-treatment. A Hierarchical clustering using all metabolites separates the control from the HAMLET-treated (15 minutes and 1 hour) samples. A majority of the metabolites measured were downregulatd (red) 1 hour after HAMLET treatment. Upregulated metabolites (green) included oleic acid and oleic acid derivatives. B Hierarchical clustering based on the significantly altered metabolites at 15 and 1 hour.
DISCUSSION
HAMLET is the first member of a new family of tumoricidal unfolded protein-lipid complexes that kill cancer cells broadly, while sparing differentiated, non-transformed cells (Hakansson et al 1995, Svensson et al 2000). In addition to its ability to kill cancer cells in vitro, HAMLET has shown therapeutic efficacy against skin papillomas and rapid topical effects on human bladder cancers (Gustafsson et al 2004a, Mossberg et al 2007). In animal models of human glioblastoma or bladder cancer, HAMLET has been shown to prolong survival and delay tumor development (Fischer et al 2004b, Mossberg et al 2010). So far, toxic effects on healthy tissues have not been detected, suggesting that HAMLET exploits conserved features of cancer cells for its activity. Some of these conserved features were identified here. The HAMLET sensitivity of cancer cells was shown to be dependent on the c-Myc oncogene expression and the glycolytic machinery especially HK1 and PFKFB1, and HIF1α, which is activated by hypoxia and oncogene activity and acts by enhancing the transcription of glycolytic enzymes, glucose transporters and LDH (Kim et al 2006, Papandreou et al 2006).
c-Myc has been called ‘’the quintessential oncogene’’ (Li et al 1984, Nilsson and Cleveland 2003) but the effects remain paradoxical, as introduction of c-Myc alone in normal cells cause cell death (Evan et al 1992). In cancer cells, c-Myc over-expression influences at least 30% if not all expressed genes, either directly or via different regulatory circuits. As HAMLET has shown activity against >40 different tumor cell types from different species, tissues and individuals, the sensitizing effect of c-Myc overexpression was expected. Tumor cells which expressed higher levels of c-Myc died more easily when exposed to HAMLET than healthy differentiated cells and knockdown of c-Myc decreased HAMLET sensitivity. The c-Myc oncogene has been proposed to promote the Warburg effect and the dependence of cancer cells on aerobic glycolysis. Thus, mutant c-Myc may make the cancer cells overly sensitive to a deregulation of glycolysis by HAMLET. In addition to c-Myc itself the Ras signaling pathway was identified as a determinant of HAMLET sensitivity by GSEA analysis and c-Myc is activated downstream of Ras thus controlling cell proliferation and survival together with other effectors such as PI3K. We have found that HAMLET causes a shift from MEK/ERK to p38 signaling in tumor cells and that p38 is involved in the early cell death response to HAMLET (Storm et al., manuscript in preparation). Healthy cells, in contrast, are characterized by low intrinsic activity in the Ras/MAPK pathway and c-Myc expression and maintain a balanced MAPK activity during HAMLET exposure, with little p38 activation, consistent with their low HAMLET sensitivity. Furthermore, the c-Myc oncogene has been proposed to influence the Warburg effect and the dependence of cancer cells on aerobic glycolysis (Dang 1999). The oncogene addiction and high metabolic activity caused by mutant c-Myc in cancer cells may make these cells overly sensitive to deregulation of glycolysis by HAMLET, further linking the effects of c-Myc and HK1 to cancer cell death.
Besides c-Myc, HIF1A, a second oncogene well-known to modulate cancer cell metabolism, was implicated in regulating the sensitivity towards HAMLET. HIF1α acts by enhancing the transcription of critical constituents of the glycolytic machinery, including glucose transporters and HK1 (Dang et al 2008). It has been proposed that HIF1 alone can drive the major metabolic changes that accompany oncogenic transformation, as identified by Otto Warburg (Denko 2008). This synergy is consistent with the HAMLET sensitizing effects of HK1 and HIF1 in the shRNA screen. In addition, deregulated expression of oncogenic c-Myc collaborates with HIF1α to confer the tumor metabolic phenotype. Paradoxically, at physiologic conditions HIF1α can inhibit the activity of c-Myc and HIF1α directly influences the expression of MXI1, which binds MAX, thereby antagonizing the effects of c-Myc (Zhang et al 2007). HIF1α also decreases c-Myc activity by attenuating c-Myc protein levels through a proteasome-dependent pathway. In contrast to HIF1α, HIF2α has been shown to stabilize the MYC-MAX complex, thereby stimulating c-Myc induced transcription. The effects of c-Myc on HAMLET sensitivity are therefore not likely to only reflect its regulation of glycolysis. In this regard, context-dependent c-Myc-mediated transcription is crucially important for tumor progression and may be so for HAMLET sensitivity as well.
The glycolytic state of tumor cells was defined as a determinant of HAMLET sensitivity by the shRNA screen and extracellular glucose levels were shown to modulate the sensitivity of tumor cells to HAMLET. Furthermore, the GSEA analysis of the shRNA screen identified several pathways related to glycolysis as important for HAMLET sensitivity. HK1 was a direct target for HAMLET, as seen by binding in a proteomic screen involving 8000 proteins and in dot blots where the purified human HK1-HAMLET interaction was confirmed. Inhibition of HK1 as well as the HK1-homologue HK2 would be predicted to reduce glucose phosphorylation, thereby creating a state similar to glucose deprivation by inhibiting glycolysis in cancer cells. The HK1/2 isoforms are associated with the mitochondrial membrane, where they control glucose utilization. In addition, HK1 decreases voltage-dependent anion channel (VDAC) conductance, thus protecting cells from cytochrome c release and apoptotic death (Abu-Hamad et al 2008). In a parallel study, we have shown that ion channel activation is a critical membrane event in HAMLET-induced cell death (Storm et al., Manuscript in preparation), raising the possibility that in addition to its effects on glycolysis, HK1 acts by modulating the interaction of HAMLET with ion channels in the mitochondrial membrane. We have also seen that peroral HAMLET treatment of APCmin mice with colon cancer modified the transcription of a number of glycolysis related proteins (Puthia et al., manuscript in preparation) suggesting relevance of these targets also in vivo.
The shRNA screen also identified PKFKB1 as a significant determinant of HAMLET sensitivity, but in contrast to HK1, PKFKB had protective effects. The mechanism behind this is not immediately obvious as the four PFKFB isoforms possess opposing kinase and bisphosphatase activities, with independent domains catalyzing the synthesis or degradation of fructose-2,6-biphosphate, respectively. The resulting effect on glycolysis is determined by the overall kinase:bisphosphatase ratio in each cell. PFKFB1, 2 and 4 have near equal kinase and bisphosphatase activities, while PFKFB3 has stronger kinase activity (740:1) and is overexpressed in cancer cells (Yalcin et al 2009). Knockdown of PFKFB1 may increase the dominance of the PFKFB3 isoform resulting in higher fructose-2,6-bisphosphate levels and enhanced glycolysis. This would be consistent with rescue after PFKFB1 knockdown, as reduced glycolysis in a cell with the Warburg phenotype appears to favor HAMLET-induced cell death.
Finally, a broad metabolomic screen demonstrated that HAMLET-treated cells rapidly loose metabolic activity. HAMLET triggered rapid and profound changes in the metabolic state of cancer cells, as shown by reverse LC-MS and GC-MS profiling. After one hour, the majority of metabolites showed a reduction in intensity, consistent with the rapid suppression of glycolysis and a resulting loss of energy required to maintain the normal metabolic machinery, especially in cancer cells. The shift was dramatic, and it is plausible that the rapid cell death response to HAMLET may result, in part, from this effect, as cells deprived of energy and metabolic activity, are known to undergo cell death (Kroemer and Pouyssegur 2008). MS/MS analysis identified metabolites that were strongly reduced, including S-adenosyl-L-homocysteine, creatine, phosphocreatine, taurine and glutathione disulfide, which are fundamental intermediates in the defense against oxidative stress (Balendiran et al 2004). Targeted GC-MS profiling identified large amounts of oleic acid in HAMLET-treated tumor cells already after 15 minutes, indicating that excess amounts of fatty acid and saturation of the fatty acid metabolism machinery might be responsible for the first, rapid response to HAMLET. Interestingly, members in the pentose-phosphate pathway (PPP) were also upregulated indicating that HAMLETs binding to HK1 might divert metabolism towards this alternative pathway. Additionally, the initial, oxidative phase of the PPP is also responsible for generation of anti-oxidant metabolites (Cairns et al 2011) and we could consistently see a depletion of important glutathione intermediates at later time points. The increased activity in the anti-oxidant pathways together with p38 activation might indicate that reactive oxygen species are important for HAMLET-induced cell death.
Interest in the metabolic changes in cancer cells, such as their higher rates of glycolysis has been reawakened by the discovery that mutations in well-known oncogenes affect cancer cell metabolism and the advent of new technologies for studying a cells metabolome. Clearly, metabolic pathways are highly interconnected with pathways that govern the classical hallmarks of cancer, such as uncontrolled proliferation and resistance to cell death. Thus, inhibition of glycolysis is becoming an important new therapeutic target. The glucose analogue 2DG has been shown to kill several cancer cell lines in vitro, to inhibit the growth of pancreatic cancer cells in nude mice and to enhance the effects of death receptor ligands, radiation and cytotoxic drugs (Coleman et al 2008, Hernlund et al 2008). Currently, 2DG is in clinical trials as single agent or in combination therapy. In addition, 3-Bromopyruvate eradicated advanced cancers in rats without apparent adverse effects (Ko et al 2004). HAMLET presents a new therapeutic option, potentially acting more broadly by inhibiting glycolysis as well as other cellular pathways regulated by c-Myc- and Ras-dependent transformation. The lack of HAMLET sensitivity in healthy, differentiated cells and lack of in vivo toxicity in different tumor models also adds to HAMLET's clinical potential.
MATERIAL AND METHODS
HAMLET-production and cell culturing
α-lactalbumin was purified from human milk and converted to HAMLET by removal of calcium and binding to oleic acid as previously described (Svensson et al 2000). For microscopy HAMLET was labeled with Alexa Fluor 568 (Invitrogen). The A498 human kidney carcinoma cell line, the A549 human lung carcinoma cell line and the Jurkat human acute T cell leukemia cell line (ATCC) were cultured as previously described (Brest et al 2007). The human renal proximal tubule cells (HRPTEC) derived from healthy adult kidney (Lonza) were grown in DMEM/F12 medium with 15 % FCS.
Cell death assays, microscopy and cell cycle analysis
HAMLET was added to the cells in serum-free medium and for longer incubations FCS was added after 1 hour. Where required cells were pre-incubated in glucose-free RPMI (Sigma or Invitrogen), 2-deoxyglucose or 3-bromopyruvate (both Sigma). After HAMLET treatment, cytotoxicity was determined by quantifying LDH release (CytoTox kit, Promega), ATP levels (ATPlite kit, PerkinElmer or CellTiter-Glo kit, Promega), remaining cell numbers or Trypan blue exclusion. For live cell microscopy, cells were seeded in a 35-mm glass-bottom dish, nuclei were stained with Hoechst 33342 (Invitrogen), Alexa Fluor 568-labeled HAMLET (21 μM) was added and the cells were examined on an inverted LSM 510 DUO confocal microscope (Carl Zeiss). For colocalization experiments, cells were seeded on 8-well chamber slides, fixed in 4 % formaldehyde permeabilized with 0.1 % Triton X-100, stained with anti-HK1 (Cell Signaling Technology) or anti-PKM2 (Milipore) antibodies followed by secondary antibodies labeled with Alexa Fluor 488. The cells were examined on an inverted LSM 510 META confocal microscope (Carl Zeiss). For cell growth inhibition, A549 cells were cultured in 0.5% FCS for 48 hours or treated with thymidine (Sigma, 2 mM in RPMI 1640 with 5% FCS) for 16 hours, followed by 16 hours in normal media and another 16 hours in thymidine-containing media (Thomas and Lingwood 1975). Cell cycle analysis was performed on a FACSCalibur device (BD Biosciences) after fixation in 80% methanol and staining with propidium iodide (Sigma).
shRNA screen
The microRNA-based shRNA library used for screening was pooled from several focused sets comprised of 10,000 shRNAs targeting approximately 3000 cancer-relevant genes. Retrovirus was prepared from packaging cells and the viral supernatant was used to infect A549 cells at single copy integration per cell. Following puromycin selection, three groups of cells each in triple replicates were used for HAMLET treatment: Mock treatment with vehicle, low dose (IC20, 7 μM), and high dose (IC80, 35 μM). After 2 hours of incubation at 37°C, genomic DNA was extracted from cell pellets and a 350 bp fragment harboring full barcode and half hairpin was amplified by PCR, purified, labeled with fluorescence dye and hybridized to the Agilent 8-plex array chips. All slides were processed following the manufacturer's instructions and scanned using an Axon 4000B scanner (Axon Instruments) at 5 micron per pixel resolution. After normalization (Smyth and Speed 2003), signals from duplicate features were averaged, values were log2-transformed and changes were scored using the MaxMean method. For the GSEA analysis the javaGSEA Desktop Application was used (http://www.broadinstitute.org/gsea/) with the c2.cp.v.25 gene set collection comparing all HAMLET-treated samples (n=8) against PBS-treated controls (n=4). P-values were derived by 10 000 permutation of the gene sets.
RNA interference and RT-PCR
c-Myc shRNA experiments were performed using Fugene HD (Roche Diagnostics) at a ratio of 4:2 with plasmids generating shRNAs to c-Myc (Genecopoeia). HK1, PFKFB1 and PKM2 siRNA (Qiagen) were transfected using Lipofectamine 2000 (Invitrogen). After 42 hours (HK1, PKM2, PFKFB1) or 72 hours (c-Myc) knockdown was examined by RT-PCR and Western blot and cells were used for viability assays. For RT-PCR RNA was prepared with the RNeasy kit (Qiagen) and real-time PCR was performed on a Rotorgene 2000 (Corbett Life Science, Sydney, Australia) or ABI 7500 instrument (Applied Biosystems) using QuantiTect Primer Assays for HK1, PFKFB1, PKM2 and PPIA (QIAGEN).
Western blot
Cells were washed with ice-cold PBS and lysed in NP-40 buffer in modified RIPA buffer or SDS buffer containing Complete protease inhibitor cocktail (Roche Diagnostics). Equal amounts of protein or, for siRNA experiments, equal volumes of lysate derived from the same number of cells were separated by SDS-PAGE and blotted onto PVDF membranes. Membranes were incubated with rabbit anti-c-Myc (Epitomics), mouse anti-Actin (Sigma-Aldrich), rabbit anti-PKM2 (Cell Signaling Technology), rabbit anti-HK1 (Milipore), rabbit anti-PFKFB1 (Abcam) or mouse anti-GAPDH antibody (Novus Biologicals) overnight at 4°C and bound antibodies were detected using corresponding HRP-conjugated secondary antibodies.
Protein array and HAMLET far-Western blot
A screen for possible interaction partners for HAMLET was performed on a ProtoArray® Human Protein Microarray v4.0 using Invitrogen's Protein-Protein Interaction Profiling Service at Invitrogen as previously described (Satoh et al 2006). Briefly, arrays were probed with Alexa Fluor 568-labelled HAMLET in 2 concentrations (5 and 50 ng/μl) in duplicate and signals were compared to a negative control array incubated without HAMLET. Proteins were defined as positive hits if the Z-score was greater than 3 standard deviations above the mean protein signal on at least one array probed with HAMLET and less than 1 standard deviation above for the control array, the Z-factor was greater than 0.5 for the corresponding array (indicating a signal 2-fold above the noise), the replicate spot CV was less than 50 % on the corresponding array and the inter-assay CV for the protein was less than 50 % on the corresponding arrays. For the HAMLET far-Western dot blot 0.5 μg human recombinant His-tagged HK1 and PKM2 (Abcam), rabbit muscle GPI and BSA (Sigma-Aldrich) were spotted onto PVDF membranes and blocked with Sat-1 and Sat-2 (Duringer et al 2003). Next, membranes were incubated overnight at 4°C with goat anti-α-lactalbumin antibodies (Bethyl Laboratories) and 2 hours at room temperature with HRP-conjugated rabbit anti-goat antibodies (Sigma-Aldrich). To quantify protein binding, dot intensity was measured with ImageJ software (Abramoff et al., 2004).
Glycolytic enzyme and lactate assays
To measure glycolytic enzyme activities, cells were treated with HAMLET, scraped into Tris-HCl buffer (pH 7.5) and homogenized by sonication. PK activity was measured as NADH consumption in a continuous spectrophotometric assay coupled to lactate dehydrogenase. HK and GPI activities were measured as NADPH formation in continuous spectrophotometric assays coupled to Glucose-6-phosphate dehydrogenase Consumption of NADH and formation of NADPH was measured by the change in absorbance at 340 nm. All enzymes and reagents were from Sigma. For the lactate assay, cells were treated with HAMLET in medium without added sodium pyruvate, the medium was collected and lactate levels measured with the Lactate Assay kit (Biovision).
Metabolomics
HAMLET-treated cells were pelleted, snap frozen in dry ice-chilled acetone and stored at −80°C. Chemicals were from Sigma or Honeywell (Morristown) and were of the highest purity. For metabolite extraction, samples were resuspended in PBS, lysed and extracted with methanol and a second time with isopropanol. LC-MS reverse phase chromatography was performed on a Zorbax C18 column with 5 μm particles at a flow rate of 20 μl/minute. Chromatography was performed as follows: equilibration in 3 % buffer B for 3 minutes, 3-50 % buffer B gradient over 5 minutes, 50-99 % buffer B gradient over 25 minutes, 99 % buffer B for 10 minutes, wash with a solution of acetone and isopropanol (1:1) for 10 minutes and re-equilibration in 3 % buffer B for 10 minutes. Data was collected using an Agilent ESIQTOF and a capillary voltage of 4000 V. Custom software written in the Matlab programming language was used to define features within each sample and compare them across samples (Smith et al., 2006). Signal intensities of quadruplicate samples were averaged and features with an abs. log2 fold change ≥ 1 and p ≤ 0.05 (Wilcoxon rank sum test) were considered to be significantly altered.
For GC-MS analysis, HAMLET-treated cells were washed with PBS followed by the addition of 650 μl of ice-cold methanol per well to stop metabolic activity. Subsequently, cells were scraped into the solvent and transferred to a reaction tube. 6 replicates consisting of 3 pooled wells each were collected per sample group. For extraction, the methanol phase was removed from the cells, dried and stored at −80°C. Cell pellets were dried in a FreeZone 2.5 lyophilizer (Labconco, Kansas City, MO), homogenized using a Mini-Beadbeater (BioSpec Products, Bartlesville, OK) and extracted with 700 μl of a pre-cooled methanol-isopropanol-water (3:3:2) mixture. The extract was then combined with the dried methanol phase from the previous step, dried again and stored at −80°C. A mixture of fatty acid methyl esters (C8, C9, C10, C12, C14, C16, C18, C20, C22, C24, C26, C28 and C30 linear chain length) was added as internal retention index markers. A solution of methoxyamine hydrochloride (40 mg/ml of 98% pure, Sigma) in pyridine (silylation grade, Pierce) was added and samples incubated at 30°C for 90 min to protect aldehyde and ketone groups. After that 1% TMSC (Pierce) in MSTFA was added and samples were incubated at 37°C for 30 min for trimethylsilylation of acidic protons. A Gerstel automatic liner exchange system with multipurpose sample MPS2 dual rail was used to inject 0.5 μl sample to a Gerstel CIS cold injection system (Gerstel). Injected samples were separated using an Agilent 6890 gas chromatograph, equipped with a 30 m long, 0.25 mm i.d. Rtx5Sil-MS column (Restek, 0.25 μm 5% diphenyl film and additional 10 m integrated guard column). Mass spectrometry was performed on a Leco Pegasus IV time of flight mass spectrometer (St. Joseph, MI) with 280°C transfer line temperature, −70 eV electron ionization and an ion source temperature of 250°C. Mass spectra were acquired from m/z 85 to 500 at 17 spectra/s and 1850 V detector voltage. Result files were processed using the metabolomics BinBase database (Fiehn et al 2005). All database entries in BinBase were matched against the Fiehn mass spectral library of ~1200 authentic metabolite spectra using retention index and mass spectrum information or the NIST05 commercial library (http://fiehnlab.ucdavis.edu/Metabolite-Library-2007/). Identified metabolites were included if present within at least 50% of the samples of each treatment group (as defined in the SetupX database (Scholz and Fiehn 2007).
Statistics
P-values were determined by paired t-test or repeated measures ANOVA with Bonferroni or Dunn multiple comparison corrections.
ACKNOWLEDGMENTS
This study was supported by the Sharon D. Lund foundation grant and the American Cancer Society, the Swedish Cancer Society, the Swedish Medical Research Council, the Medical Faculty (Lund University), the Söderberg Foundation, the Anna-Lisa and Sven-Erik Lundgren Foundation for Medical Research, the Knut and Alice Wallenberg Foundation, the Lund City Jubileumsfond, the John and Augusta Persson Foundation for Medical Research, the Maggie Stephens Foundation, the Gunnar Nilsson Cancer Foundation, the Inga-Britt and Arne Lundberg Foundation, the HJ Forssman Foundation for Medical Research, the Royal Physiographic Society, the Swedish Society for Medical Research, the Network of Excellence: EuroPathoGenomics, the Crafoord Foundation, the Österlund Foundation, the US Department of Energy Low Dose SFA Program at Berkeley Lab [DE-AC02-05CH11231], the National Institutes of Health, National Cancer Institute grant U54 CA 112970 and the California Breast Cancer Research Program [15IB-0063].
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest. There is at present no commercial development of HAMLET as a therapeutic agent in which the authors are involved.
REFERENCES
- Abu-Hamad S, Zaid H, Israelson A, Nahon E, Shoshan-Barmatz V. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J Biol Chem. 2008;283:13482–13490. doi: 10.1074/jbc.M708216200. [DOI] [PubMed] [Google Scholar]
- Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochem Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- Baran R, Reindl W, Northen TR. Mass spectrometry based metabolomics and enzymatic assays for functional genomics. Curr Opin Microbiol. 2009;12:547–552. doi: 10.1016/j.mib.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Brest P, Gustafsson M, Mossberg AK, Gustafsson L, Duringer C, Hamiche A, et al. Histone deacetylase inhibitors promote the tumoricidal effect of HAMLET. Cancer research. 2007;67:11327–11334. doi: 10.1158/0008-5472.CAN-07-1153. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R. New tools for functional mammalian cancer genetics. Nat Rev Cancer. 2003;3:781–789. doi: 10.1038/nrc1191. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Coleman MC, Asbury CR, Daniels D, Du J, Aykin-Burns N, Smith BJ, et al. 2-deoxy-D-glucose causes cytotoxicity, oxidative stress, and radiosensitization in pancreatic cancer. Free Radic Biol Med. 2008;44:322–331. doi: 10.1016/j.freeradbiomed.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- Duringer C, Hamiche A, Gustafsson L, Kimura H, Svanborg C. HAMLET interacts with histones and chromatin in tumor cell nuclei. J Biol Chem. 2003;278:42131–42135. doi: 10.1074/jbc.M306462200. [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- Fiehn O, Wohlgemuth G, Scholz M. Setup and annotation of metabolomic experiments by integrating biological and mass spectrometric metadata. Lecture Notes in Computer Science. 2005;3615:224–239. [Google Scholar]
- Fischer W, Gustafsson L, Mossberg AK, Gronli J, Mork S, Bjerkvig R, et al. Human alpha-lactalbumin made lethal to tumor cells (HAMLET) kills human glioblastoma cells in brain xenografts by an apoptosis-like mechanism and prolongs survival. Cancer Res. 2004a;64:2105–2112. doi: 10.1158/0008-5472.can-03-2661. [DOI] [PubMed] [Google Scholar]
- Fischer W, Gustafsson L, Mossberg AK, Gronli J, Mork S, Bjerkvig R, et al. Human alpha-lactalbumin made lethal to tumor cells (HAMLET) kills human glioblastoma cells in brain xenografts by an apoptosis-like mechanism and prolongs survival. Cancer research. 2004b;64:2105–2112. doi: 10.1158/0008-5472.can-03-2661. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Leijonhufvud I, Aronsson A, Mossberg AK, Svanborg C. Treatment of skin papillomas with topical alpha-lactalbumin-oleic acid. N Engl J Med. 2004a;350:2663–2672. doi: 10.1056/NEJMoa032454. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Leijonhufvud I, Aronsson A, Mossberg AK, Svanborg C. Treatment of skin papillomas with topical alpha-lactalbumin-oleic acid. N Engl J Med. 2004b;350:2663–2672. doi: 10.1056/NEJMoa032454. [DOI] [PubMed] [Google Scholar]
- Hakansson A, Zhivotovsky B, Orrenius S, Sabharwal H, Svanborg C. Apoptosis induced by a human milk protein. Proc Natl Acad Sci U S A. 1995;92:8064–8068. doi: 10.1073/pnas.92.17.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hernlund E, Ihrlund LS, Khan O, Ates YO, Linder S, Panaretakis T, et al. Potentiation of chemotherapeutic drugs by energy metabolism inhibitors 2-deoxyglucose and etomoxir. Int J Cancer. 2008;123:476–483. doi: 10.1002/ijc.23525. [DOI] [PubMed] [Google Scholar]
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Ihrlund LS, Hernlund E, Khan O, Shoshan MC. 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Mol Oncol. 2008;2:94–101. doi: 10.1016/j.molonc.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004;324:269–275. doi: 10.1016/j.bbrc.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Li Y, Holland CA, Hartley JW, Hopkins N. Viral integration near c-myc in 10-20% of mcf 247-induced AKR lymphomas. Proc Natl Acad Sci U S A. 1984;81:6808–6811. doi: 10.1073/pnas.81.21.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathupala SP, Rempel A, Pedersen PL. Aberrant glycolytic metabolism of cancer cells: a remarkable coordination of genetic, transcriptional, post-translational, and mutational events that lead to a critical role for type II hexokinase. J Bioenerg Biomembr. 1997;29:339–343. doi: 10.1023/a:1022494613613. [DOI] [PubMed] [Google Scholar]
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Mossberg AK, Wullt B, Gustafsson L, Mansson W, Ljunggren E, Svanborg C. Bladder cancers respond to intravesical instillation of HAMLET (human alpha-lactalbumin made lethal to tumor cells). Int J Cancer. 2007;121:1352–1359. doi: 10.1002/ijc.22810. [DOI] [PubMed] [Google Scholar]
- Mossberg AK, Hou Y, Svensson M, Holmqvist B, Svanborg C. HAMLET treatment delays bladder cancer development. J Urol. 2010;183:1590–1597. doi: 10.1016/j.juro.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- Satoh J, Nanri Y, Yamamura T. Rapid identification of 14-3-3-binding proteins by protein microarray analysis. J Neurosci Methods. 2006;152:278–288. doi: 10.1016/j.jneumeth.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Scholz M, Fiehn O. SetupX--a public study design database for metabolomic projects. Pac Symp Biocomput. 2007:169–180. [PubMed] [Google Scholar]
- Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JM, Li MZ, Chang K, Ge W, Golding MC, Rickles RJ, et al. Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet. 2005;37:1281–1288. doi: 10.1038/ng1650. [DOI] [PubMed] [Google Scholar]
- Silva JM, Marran K, Parker JS, Silva J, Golding M, Schlabach MR, et al. Profiling essential genes in human mammary cells by multiplex RNAi screening. Science. 2008;319:617–620. doi: 10.1126/science.1149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK, Speed T. Normalization of cDNA microarray data. Methods. 2003;31:265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- Soucek L, Whitfield J, Martins CP, Finch AJ, Murphy DJ, Sodir NM, et al. Modelling Myc inhibition as a cancer therapy. Nature. 2008;455:679–683. doi: 10.1038/nature07260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg C, Agerstam H, Aronson A, Bjerkvig R, Duringer C, Fischer W, et al. HAMLET kills tumor cells by an apoptosis-like mechanism--cellular, molecular, and therapeutic aspects. Adv Cancer Res. 2003;88:1–29. doi: 10.1016/s0065-230x(03)88302-1. [DOI] [PubMed] [Google Scholar]
- Svensson M, Hakansson A, Mossberg AK, Linse S, Svanborg C. Conversion of alpha-lactalbumin to a protein inducing apoptosis. Proc Natl Acad Sci U S A. 2000;97:4221–4226. doi: 10.1073/pnas.97.8.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DB, Lingwood CA. A model of cell cycle control: effects of thymidine on synchronous cell cultures. Cell. 1975;5:37–42. doi: 10.1016/0092-8674(75)90089-6. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Watson PH, Pon RT, Shiu RP. Inhibition of c-myc expression by phosphorothioate antisense oligonucleotide identifies a critical role for c-myc in the growth of human breast cancer. Cancer research. 1991;51:3996–4000. [PubMed] [Google Scholar]
- Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. discussion 3080. [DOI] [PubMed] [Google Scholar]
- Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, et al. An oncogenomics- based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852–864. doi: 10.1016/j.cell.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]