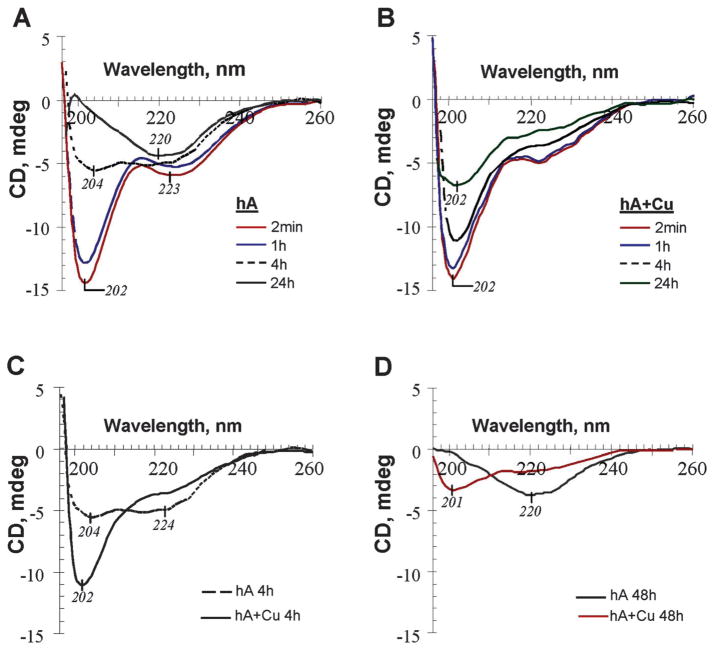
Fig. 4.
Cu2+ inhibits human amylin secondary conformational transitions in solution. (A–D) Comparative far-UV CD data for the conformational changes of human amylin (20 μM) in PBS alone or in the presence of equimolar amount of Cu2+. In the presence of Cu2+, human amylin conformational transitions to a β-sheet-rich structure are blocked.