Figure 1.
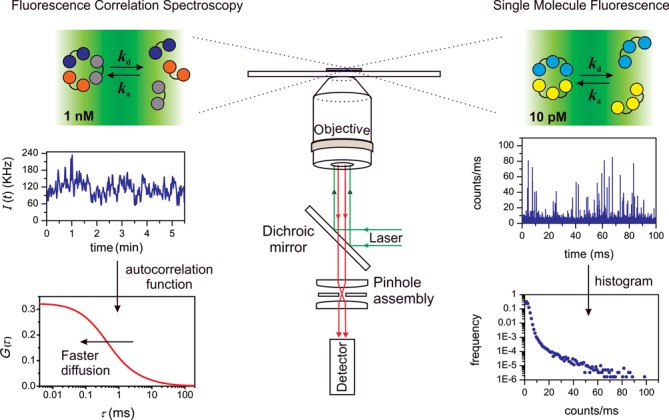
Experimental setup. We investigated the oligomerization equilibrium and dynamics of PCNA (a trimer) and β (a dimer) using FCS and single molecule fluorescence measurements. Experiments were performed in a confocal single-molecule setup. FCS decays were acquired with 1 nM labeled and variable concentrations of unlabeled protein. Fluctuations in fluorescence intensity (middle left) were analyzed in terms of the autocorrelation function (bottom left), which shifts toward faster times as the oligomeric proteins dissociate into faster-diffusing monomers. Single-molecule traces (middle right) were measured with 10 pM labeled protein solutions. Bursts of fluorescence are generated when individual fluorescent molecules traverse the observation volume. Single-molecule data was analyzed in terms of histograms representing the frequency of bursts containing k photons per millisecond (bottom right).
