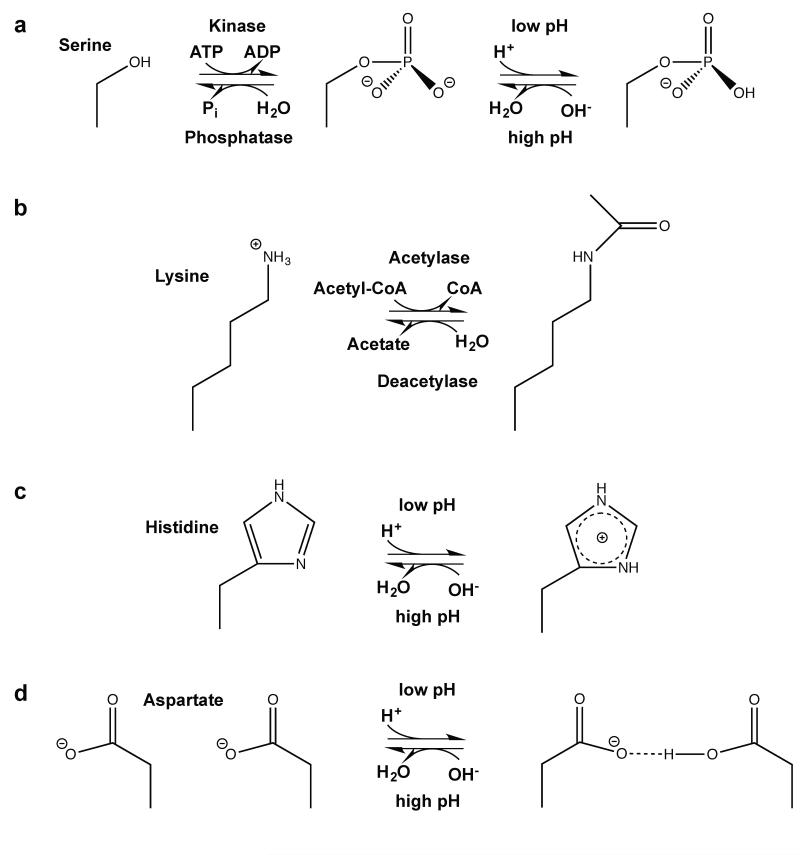
Figure 1.
Examples of amino acid post-translational modifications associated with changes in charge. (a): Phosphorylation of e.g. serines (shown), threonines, tryptophans and histidines leads to addition of a negative charge at weakly basic conditions (151). (b): Lysine acetylation shields the lysine amino group e.g. to decrease the affinity to DNA (126), (c): Histidines can quickly abstract protons to shuttle protons or to function as a pH sensor site, (d): When pKa values are upshifted, protonation of carboxyl groups of glutamate or aspartate (shown) can lead to formation of new hydrogen bonds important for conformational changes of proteins.