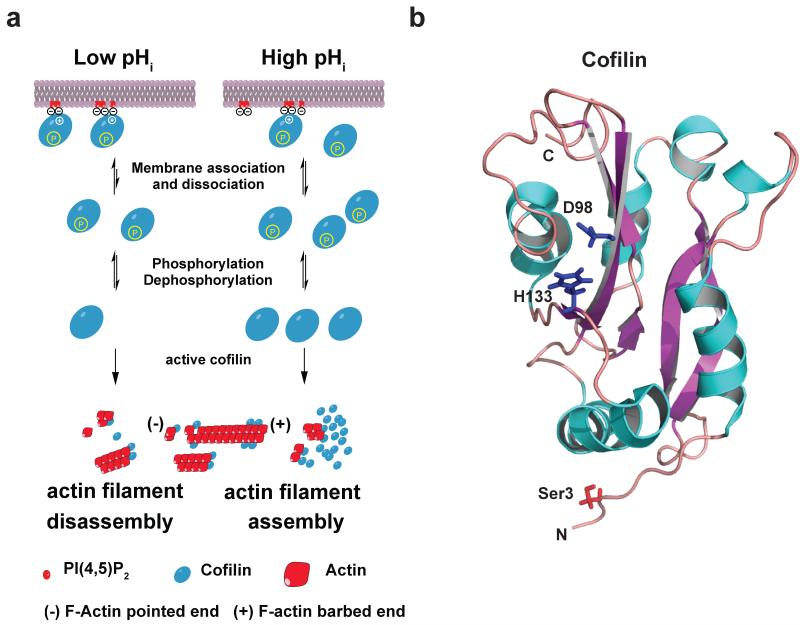
Figure 3.
Coincidence regulation of cofilin: (a) Model for coincidence detection of cofilin near the plasma membrane. At lower pHi < 7.2, the affinity of cofilin is higher, increasing binding to plasma membrane PI(4,5)P2 (red). At higher pHi > 7,2, less cofilin is bound to PI(4,5)P2, increasing the cytosolic pool of active cofilin if Ser3 is dephosphorylated. At higher pHi actin assembly is increased partly due to higher cofilin concentration. (b) Cartoon representation structure of human cofilin structure (pdb code: 1q8x) (109). The five α-helices are colored blue and the six β-sheets are colored purple. Side chains of Ser3, Asp98 and His133 are shown. N-terminal Ser3 is modified by phosphorylation. A salt bridge between Asp98 and His133 is formed under slight acidic conditions. His133 closely interacts with PI(4,5)P2 when doubly protonated (34).