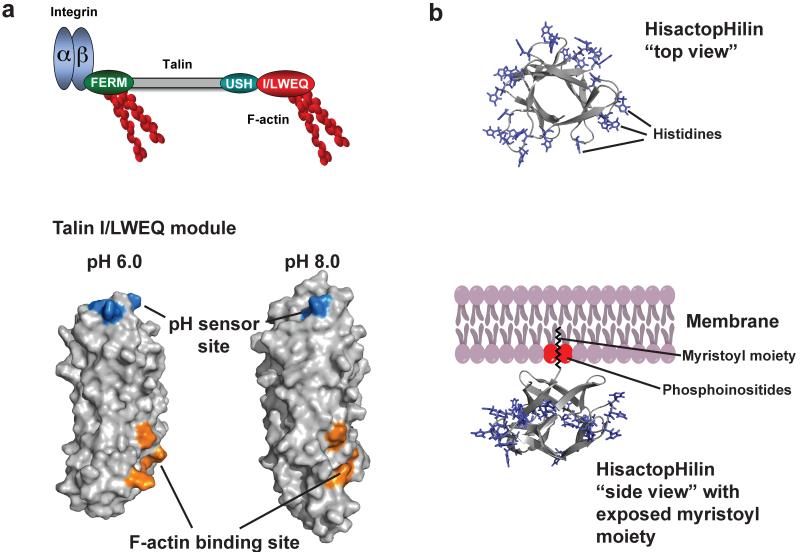
Figure 4.
Allosteric regulation of talin-actin binding by pH. (a) Domain organization of talin (upper panel), including an N-terminal FERM (domain that binds the β-subunit of integrin receptors and F-actin, a central rod domain (gray) and a C-terminal I/LWEQ (red) actin binding module that binding F-actin. F-actin binding by the I/LWEQ module but not by the FERM domain is pH sensitive with more binding at lower pH. Lower panel shows cartoon representation of C terminal actin-binding domain of talin. Residues E2337, E2342, H2418, D2482 form a pH sensor that induces pH-dependent conformations that allow actin binding only at lower pH (b) Cartoon representation of the histactopHilin structure and model of membrane attachment of hisactopHilin (pdb code: 1hcd). “Top view” shows that all histidines in loops in turns are equally distributed around the protein. However, viewed from the “side” these histidines point towards the cytoplasm, while the β-sheets point towards the membrane when the N-terminal myristoyl moiety is exposed partly by PI(4,5)P2.