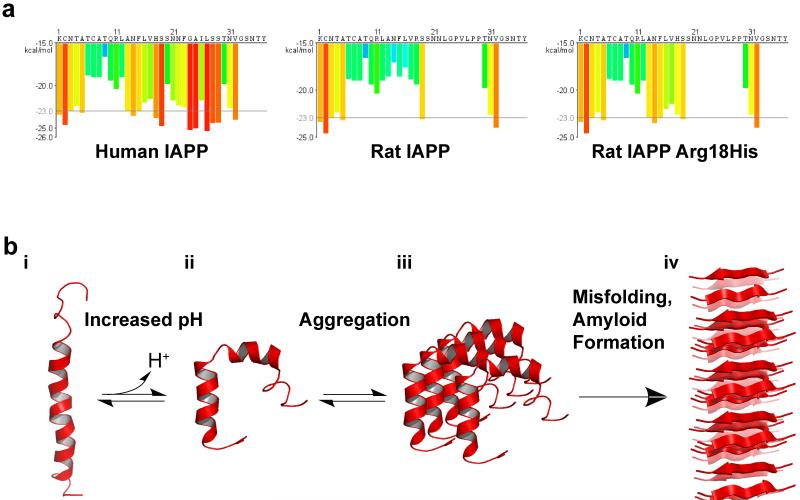
Figure 5.
Structural Regulation of hIAPP by pH. (a) IAPP sequences and segment propensity for fibril formation. The predicted energy for fibrillation of every six-residue segment of human IAPP, rat IAPP, and rat His18Arg IAPP are shown. Warmer colors represent a greater propensity for fibrillation, with red histogram bars represent hexapeptides that are predicted to form fibrils. Due to variations in the sequence, human IAPP has a much higher propensity to form fibrils than mouse IAPP. Mutation of Arg18 to His increases the propensity of rat IAPP to form fibrils. Graphs were generated using ZipperDB (http://services.mbi.ucla.edu/zipperdb/)(41) and modified from (153). (b) Potential model of pH dependent amyloid formation by IAPP. i) hIAPP, located in the insulin secretory granules, is bound to insulin b-chain (not shown) or to the membrane surface in an extended kinked helix conformation. ii) As IAPP is released from the acidic environment of the vesicle to the neurtral pH of the cytoplasma or extracellular space, His18 becomes deprotonated, weakening the insulin-hIAPP interaction and promoting insertion of hIAPP in the membrane by inducig a change in conformation. iii) Membrane associate hIAPP aggregates and, iv) undergoes further conformational changes leading to the formation of β-sheet-rich amyloid fibrils. Low pH structure: 2KB8 (103). Neutral pH structure: 2L86 (97). In both structures, the N terminus is located at the bottom of the figure. Figure adapted from (97; 150)