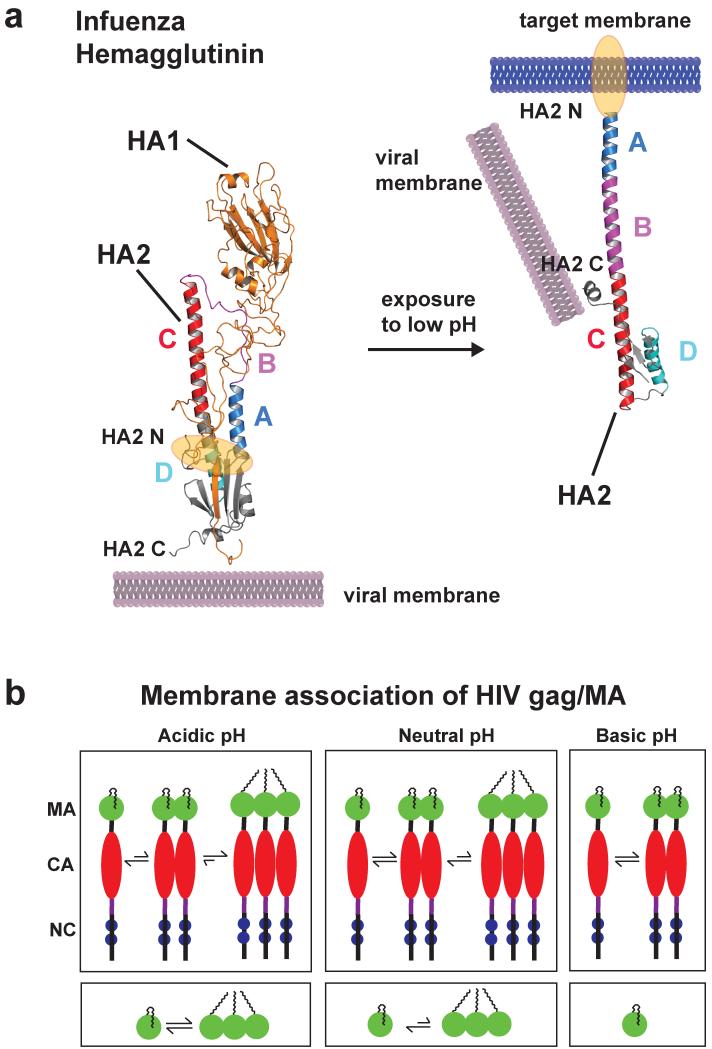
Figure 6.
Low pH induces a large conformational change of the influenza HA2 protein (only one monomer is shown). (a) The structure of prefusion complex (left) of HA1 (gold, pdb code: 2hmg) and HA2 (multicolor, pdb code: 1htm) and the HA2 at the fusion pH (right) structure are shown as cartoon representations. In the prefusion complex the HA2 has a metastable conformation, and HA1 acts as a clamp to keep HA2 in this conformation. The hydrophobic fusion peptide (highlighted in orange) is buried in a hydrophobic core distant from the tip of the protein complex. Low pH leads to protonation of several charged residues throughout HA1 and HA2, and HA1 dissociates partly. This leads to a spontaneous conformational change of region B (magenta) that becomes an α-helix to form a new continuous helix A (blue), B and C (red). The fusion peptide moves ~100 Å from the core to the tip for insertion into the target membrane. Helix D (cyan) now packs against helix A, and a new hydrophobic core is formed. The C-terminus moves more towards the new N terminus and has more flexibility important for membrane fusion. (b) Schematic representation showing multimerization events of HIV gag and MA proteins as a function of pH (MA: matrix, CA: capsid, NC: nucleocapsid). Decreasing pH promotes myristoyl exposure membrane targeting and formation of multimers of both gag and MA protein, critical mediators for HI virus assembly. Structure figure reprinted with permisson from Jamil S. Saad (33).