Table 1.
Simultaneous arming/SAR studies of natural products and drugs by amination and aziridination with sulfamate 9.
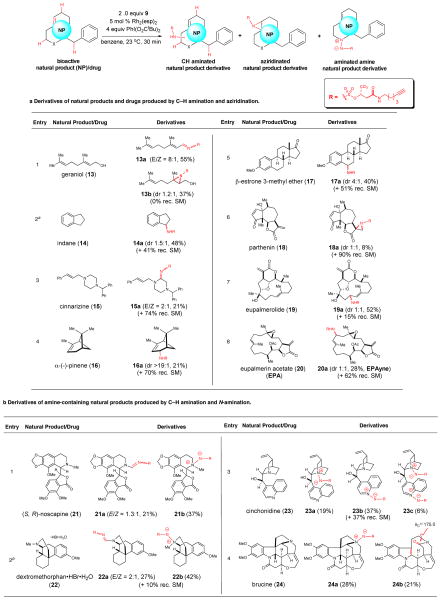
|
Diastereomers can be generated with achiral substrates, as in this case with indane, since the sulfamate reagent bears a stereogenic center.
C–H amination of dextromethorphan•HBr monohydrate was performed in the presence of K2CO3 (6.0 equiv). (rec. SM = recovered starting material)
