Abstract
Background
Currently, osteochondral allografts (OCA) are typically used after 4°C storage for prolonged durations (15-43days), which compromises chondrocyte viability, especially at the articular surface. The long-term in vivo performance of these fresh-stored allografts, in association with variable cellularity, is unknown.
Hypothesis/Purpose
Determine the effect of 4°C storage duration (14, 28days) versus the best (fresh) and worst (frozen) conditions of chondrocyte viability on structure, composition, and function of cartilage in the goat, and the association of retrieved chondrocyte cellularity with those tissue properties.
Study Design
Controlled Laboratory Study
Methods
The effect of allograft storage on in vivo repair outcomes was determined for OCA transplanted into fifteen recipient goats and analyzed at 12months. Repair outcomes were assessed by examining cartilage structure (gross, histopathology), composition (cellularity by depth, matrix fixed charge), and biomechanical function (stiffness). Relationships between cellularity and structural scores, matrix fixed charge, and stiffness were assessed by linear regression.
Results
Repair outcomes in 4°C-stored OCA were inferior to fresh OCA, and were accompanied by diminished cellularity at the surface, matrix fixed charge, and histopathological structure. Overall, cellularity by depth and matrix fixed charge in cartilage of fresh OCA were similar to non-operated controls. However, cellularity at the articular surface and matrix fixed charge in 4°C-stored OCA were lower than fresh, by ~55% (95%CI, 32-76%) and ~20% (95%CI, 9-30%), respectively. In frozen OCA, cellularity and matrix fixed charge were lower than 4°C-stored OCA, by ~93% (95%CI, 88-99%) and ~22% (95%CI: 10-35%), respectively. Cellularity correlated negatively with cartilage health indices, including structural scores, and positively with matrix fixed charge and stiffness.
Conclusion
Reduced cellularity at the articular surface, resulting from 4°C storage, was associated with variable long-term outcomes, versus consistently good repair by fresh allografts. Cellularity at the articular surface was an important index of biological performance.
Clinical Relevance
Normal chondrocyte density in vivo, especially in the superficial region of cartilage, is important for maintaining long-term cartilage function and matrix content. In human cartilage, containing cells at ~3-5× lower density than goat, repair outcomes may be related to absolute minimum number of cells rather than density.
Keywords: fresh-stored osteochondral allografts, cartilage repair, animal models, chondrocyte viability
Introduction
Osteochondral allografting is a desirable treatment option to repair large articular defects, providing functional restoration of the affected joint. Traditionally, osteochondral allografts were implanted fresh, within 7days, and have high chondrocyte viability at implantation. Usage of such fresh allografts has resulted in long-term clinical success rates exceeding 75%.4,7,9,12,24 Currently, osteochondral allografts are stored at 4°C to accommodate commercial screening and processing protocols, with testing typically requiring at least 14days.1,20,45 Such fresh-stored osteochondral allografts are routinely used after 4°C storage for prolonged durations (15-43days), which compromises chondrocyte viability, and has resulted in some short-term clinical improvement compared to pre-operative function for small cohorts of patients.11,20,23,45 However, long-term in vivo performance of these fresh-stored allografts, in association with variable cellularity, is unknown.
The long-term efficacy of osteochondral allografts is thought to be due to the presence of viable chondrocytes within graft cartilage which preserves tissue homeostasis and prevents degeneration. Preserving chondrocyte viability during allograft insertion, as well as storage, may be critical to graft function. Significant impaction forces can sometimes be generated during insertion of osteochondral allografts, and such supra-physiologic loading and other traumatic injury has deleterious effects on chondrocyte viability, especially at the articular surface.6,10,15,19,30,33,39,42 In addition, during prolonged 4°C storage, chondrocytes, particularly in the superficial zone of cartilage, succumb with increasing storage duration; chondrocyte viability deteriorates substantially in the superficial zone by 14days,3,26 and overall by 28days.3,26,31,32,40,43,44,47 While shorter 4°C storage durations (i.e. 14days) preserve overall chondrocyte viability, acquisition and distribution of these allografts is logistically challenging. Significant loss of viable chondrocytes at longer 4°C storage durations (i.e. 28days) may contribute to graft degeneration and subsequent failure, but the increased storage duration allows for easier distribution of allografts. Investigating the relationship between these critical storage durations and repair outcomes may expand/limit the definition of suitable graft tissue.
Determinants of long-term allograft function can be pursued using multi-scale analyses of cartilage repair in animal models. In such animal models, allografts stored at 4°C for 14days appear grossly, biochemically, and biomechanically similar to fresh allografts at short post-operative durations up to 3months,25 but exhibit mild to moderate gross degenerative changes at 6 and 12months.34,37 Allografts stored at 4°C for >21days have variable outcomes; some grafts tend to undergo severe degeneration, while others tend to be similar in appearance to fresh grafts and grafts stored at 4°C for 14days.21,34 At 12months, some 4°C stored allografts appear qualitatively to have diminished cellularity at the cartilage surface,34 which may be an important predictor of long-term graft efficacy. While storage duration did not correlate with histologic appearance at 12months,34 other determinants of repair outcomes have not been investigated. Quantitative analysis of cellularity in retrievals and its relationship to graft efficacy may expand the current understanding of in vivo maintenance of cartilage structure, composition, and function.
Thus, the hypothesis of this study was that decreased chondrocyte viability in stored osteochondral allografts is associated with poor repair outcomes. The specific aims were to determine the effect of allograft storage on the macroscopic structure, cellular and matrix fixed charge composition, and biomechanical function of cartilage within osteochondral allografts retrieved at 12months in a goat model, and to assess the associations between chondrocyte cellularity and cartilage structure, matrix fixed charge, and biomechanical stiffness. The results of this study will provide a comparison between allografts stored at 4°C for critical durations (14 and 28days) versus the best (fresh) and worst (frozen) conditions of chondrocyte viability within allografts.
Materials and Methods
Experimental Design
Adult Boer goats (n=16, 2-3yo) were operated (OP) in one knee with IACUC approval. Each OP knee received two site-matched osteochondral allografts of different storage treatments (FROZEN, FRESH, stored at 4°C for 14days or 28days) implanted into alternating medial femoral condyle (MFC) and lateral trochlea (LT) sites. Contralateral knees were Non-Operated (Non-OP) controls. At 12months, animals were euthanized and both knees analyzed, assessing macroscopic and histologic structure, cellular and matrix composition, and biomechanical function.
Donor Preparations
Donor osteochondral allografts were prepared from both knees of adult Boer goats (n=8, 2-4yo). Hind limbs were received on wet ice within 24hours of sacrifice. Under aseptic conditions, each knee was harvested and divided into condyle and trochlea fragments. Fragments were thoroughly rinsed with phosphate-buffered saline (PBS) supplemented with antibiotics-antimycotics (100U/mL penicillin, 100μg/mL streptomycin, and 0.25μg/mL fungizone, PSF). Some fragments (n=8) were stored FROZEN at −70°C for 10days. Other fragments (each, n=8) were stored at 4°C in tissue culture medium (low-glucose Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, 0.1mM non-essential amino acids, 2mM L-glutamine, 25μg/mL L-ascorbic acid, and PSF) for 3days (FRESH), 14days (4°C/14d), or 28days (4°C/28d).
Allograft Surgery
Osteochondral allografting was performed through a medial knee arthrotomy under general anesthesia, as previously described.27 Briefly, the knee joint was exposed through a medial parapatellar incision, and lateral patella dislocation. An osteochondral defect (d=7.5mm, h=5mm) was created at MFC and LT sites under continuous saline irrigation. An orthotopic cylindrical osteochondral plug (d=8mm, h=5mm) was harvested from donor tissue, thoroughly cleansed with saline, and inserted carefully into the defect with gentle impaction until the graft was flush with the surrounding articular surface, using a graft tamp and load-cell instrumented mallet. Following closure, OP knees were cast in a modified Thomas-Schroeder splint for 13days to limit weight-bearing.
Animals were monitored post-operatively for pain and lameness throughout the study. After 12months, animals were euthanized with potassium chloride (1-2mmol/kg) intravenously under stage III anesthesia. One animal was sacrificed at 2months due to a lateral luxated patella, and analysis was not included in the study.
Surgical Graft Insertion
Graft insertion parameters, including impaction force (peak and average), impulse (average and total), tap duration, and number of taps, were measured using a mallet instrumented with a dynamic force sensor (208C05, PCB Electronics, Depew, NY) to allow loads to be recorded during gentle mallet impaction.6 Force-time data was acquired at 50 kHz, and processed to determine impaction parameters. The loading force (N) was taken as the peak or average of all taps, the magnitude of the load impulse (N·s) was calculated as the area under the force-time curve for each tap and all taps, and the impact duration (ms) was calculated as the average interval between half-maximum loads.
Repair Site Analysis
Intact knee joints were received on wet ice within 24hours of sacrifice. Distal femurs were harvested, photographed, and examined grossly. Next, osteochondral cores (d=15mm, h~8-10mm) were isolated to encompass the experimental site, using a custom coring bit under irrigation with cold PBS+PSF. Samples were tested biomechanically, and then osteochondral slabs (width=2mm), cut centrally from proximal to distal, were isolated. Osteochondral slabs were fixed in 4% paraformaldehyde, and then analyzed for cellularity, matrix fixed charge, and histopathology index.
Macroscopic Structure
Macroscopic structure of repair tissue was evaluated grossly for surface texture, defect filling/size, and graft-recipient cartilage integration. The gross-score had a 0-9 scale, with high scores corresponding to degeneration and/or poor repair.13 Scores were averaged from two blinded observers. Surface texture was scored for the central region of the repair tissue, from normal (score=0) to <50% normal (score=3). Defect filling/size was scored based on surface congruity and repair size, as normal/flush with d~8mm (score=0), flush with 5mm≤d<8mm (score=1), flush with d<5mm or depressed with 5mm≤d<8mm (score=2), and depressed with d>5mm (score=3). Graft-recipient cartilage integration was scored based on transition across the host and repair region, from complete integration (score=0) to <50% smooth transition (score=3).
Cartilage Composition
Cellularity was assessed with depth from the articular surface. Cell nuclei were labeled fluorescently, and full-thickness cartilage was imaged along a vertical profile to a depth of 45μm at 1.5μm intervals by confocal microscopy (Leica TCS SP5, Buffalo Grove, IL). Image stacks were processed in three-dimensions with a custom routine to localize and count cells.17 The articular surface was segmented manually from a single background image, which clearly delineated the boundaries of cartilage. Cellularity was calculated as the number of cells divided by cartilage volume (cells/cm3) from the segmented articular surface (by 50 or 100μm intervals). Cellularity at selected depths (0-50, 51-200, 201-600μm) from the articular surface was reported, and results at all depths are in the Appendix. Representative cellularity images were illustrated by merging five fluorescent images from the stack (at 0, 7.5, 15, 22.5, and 40.5μm) to form one single image. For correlations, cellularity was calculated for superficial, middle, and deep zones of cartilage, defined as 0-15%, 15-50%, and 50-100% of the cartilage thickness.
Matrix fixed charge in cartilage was assessed by Hexabrix-Enhanced micro-computed tomography (HE-μCT), as previously described.27 Hexabrix, an ionic contrast agent, distributes inversely to the fixed charge density in soft tissues, and is therefore a sensitive (inverse) indicator of proteoglycan content.29 Briefly, fixed osteochondral slabs were incubated for ~1day in a Hexabrix (20%, by volume in PBS) solution, and then imaged in 3D using μCT (Skyscan 1076, Kontich, Belgium): 70kV, voxel=(18μm)3. HE-μCT value, expressed as percent, was determined by comparing the imaged x-ray attenuation value to Hexabrix phantoms, fit by linear regression, within the segmented cartilage region (width=2mm). The spatial variation in matrix fixed charge was illustrated with color maps representing 0-20% HE-μCT value within the cartilage.
Cartilage histopathology was assessed with the modified Mankin score38 using Safranin-O and Hematoxylin/Eosin (H&E) stained slides because osteochondral allograft repair resembles normal hyaline cartilage structure at the time of implant. Previously fixed osteochondral slabs were decalcified, embedded in paraffin, sectioned at 7μm, deparaffinized, stained, and digitized at 20X (Leica SCN400, Buffalo Grove, IL). The histopathology-score had a 0-15 scale, with high scores corresponding to degeneration. Scores were averaged from two blinded observers.
Biomechanical Function
Cartilage load-bearing function was assessed by indentation testing at the center of each osteochondral core. Using a benchtop mechanical tester (v500cs, Biomomentum Inc., Canada), samples were compressed rapidly by 100μm, at three sites 0.5mm apart (proximal to distal).22 The peak load was divided by the indentation depth, normalized to cartilage thickness and indenter tip area, and three points averaged to determine the indentation material stiffness, expressed in units of MPa.
Statistical Analysis
Data are presented as mean±SEM. For each Non-OP knee (n=15), metrics at MFC and LT (except cartilage stiffness and thickness) were averaged. The effect of allograft storage (Non-OP, FROZEN, FRESH, 4°C/14d, 4°C/28d) on cartilage cellularity and HE-μCT value, were determined by ANOVA. For cellularity, depth (0-50μm, 51-200 μm, 201-600μm) from the articular surface was considered a repeated measure, and a 2-way repeated measures ANOVA was performed. For metrics (i.e. cartilage stiffness and thickness) that varied at MFC and LT in Non-OP, effect of allograft storage and site (MFC, LT) were determined by 2-way ANOVA. Tukey post-hoc comparisons were performed to compare treatments with significant differences (p<0.05). For nonparametric data (i.e. gross- and histopathology-scores), samples were analyzed analogously using Kruskal-Wallis and Dunn’s tests. Power analysis (α=0.05 and 1-β=80%) was performed with chondrocyte cellularity as the primary endpoint, and n=8 samples per treatment group was determined to detect a difference of 80% of the SD, an effect size corresponding to a 40% reduction of superficial chondrocyte viability in 4°C/28d stored osteochondral allografts versus fresh controls.26 For secondary endpoints where effect size may be smaller or which differed at sites, power may not be sufficient to detect significant differences.
The relationships between indices of cartilage health and chondrocyte cellularity were assessed by regression. Gross- and histopathology-score were correlated with cellularity by nonparametric Spearman’s rank method.14 HE-μCT value (inverse of matrix fixed charge) and cartilage stiffness were correlated with cellularity by linear regression. Coefficients of determination, R2 (parametric) and ρ2 (nonparametric), are reported for significant relationships (p<0.05).
Results
Graft insertion parameters (mean±SD), including impaction force, impulse, tap duration, and number of taps, did not vary with allograft storage (p>0.4). Allografts were inserted with 36±20 taps, for a total impulse of 6.77±3.35N·s. For graft insertions, each tap was moderate, with peak and average forces of 415±14N and 197±7N, respectively, and impulse of 0.20±0.03N·s for 2.71±3.93ms duration. Two grafts required 96 and 112 taps, which is about three standard deviations away from the mean number of taps.
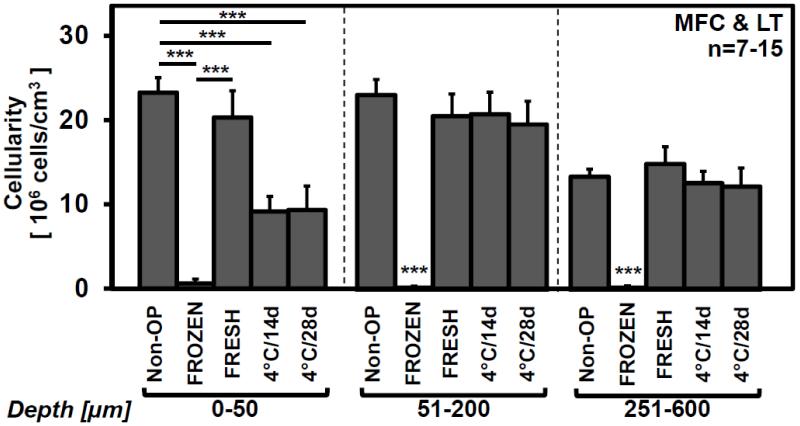
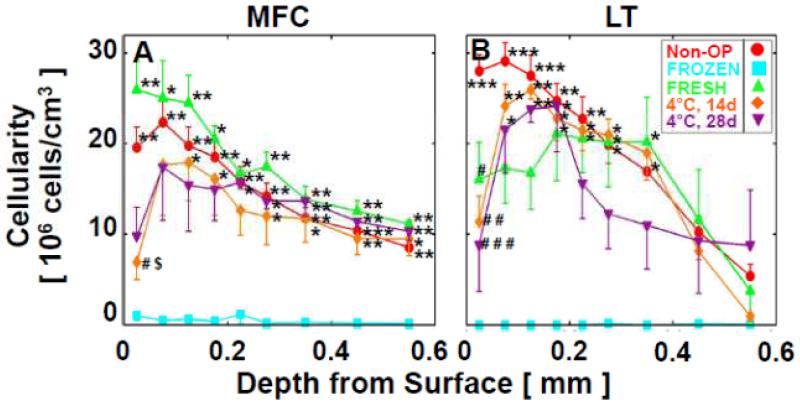
Quantitatively, chondrocyte cellularity varied with allograft storage (p<0.001), with depth from the articular surface (p<0.001), and with a significant interaction effect (p<0.001). Cellularity in Non-OP was similar to FRESH (p>0.9) decreasing throughout the cartilage depth (see Appendix Fig. 1), and was 23.7±1.7×106cells/cm3 for depth=0-50μm, 23.0±1.8×106cells/cm3 for depth=51-200μm, and 13.3±0.9×106cells/cm3 for depth=201-600μm from the articular surface (Fig. 1). At the articular surface for depth=0-50μm, cellularity in 4°C/14d and 4°C/28d stored allografts was ~60% lower than Non-OP (95% Confidence Interval [CI]: 49-72%; each, p<0.001), and tended to be ~55% lower than FRESH (95%CI: 32-76%; each, p=0.07). At depth=51-200μm and 201-600μm, cellularity in 4°C/14d and 4°C/28d stored allografts was similar to Non-OP and FRESH (each, p>0.7), but ~98% higher than FROZEN (95%CI: 95-100%; p<0.001 vs. all storage treatments).
Figure 1.
Effect of in vivo allograft storage on cellularity at selected (0-50, 51-200, 201-600μm) depths from the articular surface in retrieved osteochondral allografts after 12months. Mean±SEM. ***p<0.001. See Appendix Figure 1 for detailed cellularity by depth.
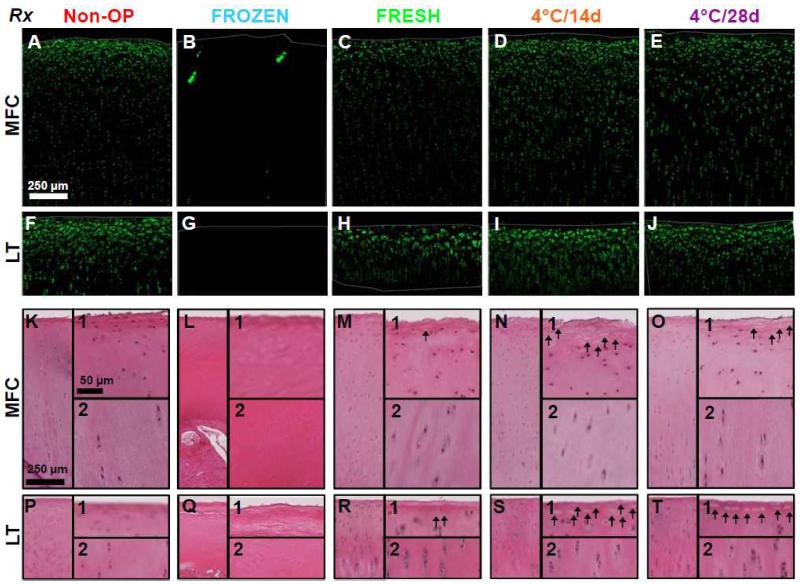
Representative confocal images and histological sections of cartilage within the graft region agreed qualitatively with the above quantitative cellularity data. Non-OP and FRESH allografts had typical chondrocyte organization, with flattened cells at high density near the articular surface and columns of cells at lower density in the deeper regions of cartilage (Fig. 2A,C,F,H,K,M,P,R). In 4°C/14d and 4°C/28d stored allografts, cellularity was diminished at the articular surface, but similar to Non-OP and FRESH in deeper regions of cartilage; confocal images lacked flattened cells (Fig. 2D-E,N-O), and histological sections showed empty cellular lacunae at the articular surface (Fig. 2N1-O1,S1-T1). FROZEN allografts were acellular in both confocal images and histological sections (Fig. 2B,G,L,Q).
Figure 2.
Qualitative cellularity analysis in retrieved allografts. Representative (A-J) confocal and (K-T) H&E histology images of vertical profile view throughout the depth of articular cartilage of retrieved osteochondral allografts at (A-E,K-O) MFC and (F-J,P-T) LT sites after 12months in vivo. H&E histology images depict the full-profile and zoomed regions of the (1) surface, and (2) deep zone. Thin gray lines denote the cartilage surfaces. Arrows indicate empty cellular lacunae.
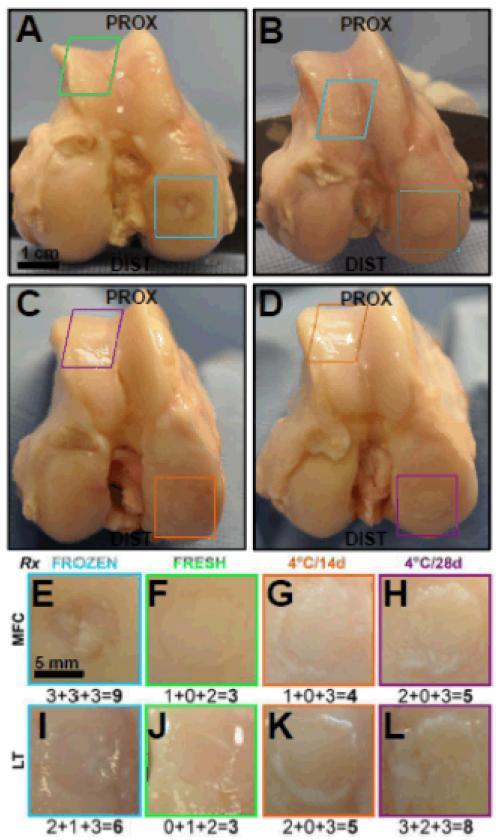
Gross-score varied with allograft storage (p<0.001). Overall joints appeared macroscopically normal, with no osteophyte formation or extensive degeneration (Fig. 3A-D). Non-OP gross-score was 0.3±0.1, and was lower than all other allograft storage treatments (each, p<0.001, Table 1). Gross-score in FRESH was consistently low at MFC (range 2-3, Fig. 3F), but was more variable at LT (range 3-8, Fig. 3J) and similar to 4°C/14d (range 2-7) and 4°C/28d (range 3-9) stored allografts (Fig. 3G-L, p>0.9). Gross-score was 7.4±0.7 in FROZEN (Fig. 3E-I), and higher than FRESH, 4°C/14d, and 4°C/28d (p<0.01 vs. FRESH, p<0.001 vs. 4°C/14d, p<0.05 vs. 4°C/28d, Table 1). Surface texture was similar among FRESH, 4°C/14d, and 4°C/28d (p>0.6). Defect filling/size tended to be worse in 4°C/28d versus 4°C/14d stored allografts (p=0.1), but still better than in FROZEN (p<0.05). All implanted allografts remained clearly demarcated from the surrounding host cartilage at retrieval; 4°C/14d and 4°C/28d stored allografts had worse cartilage integration scores than FRESH at MFC (each, p<0.01, Appendix Table 1).
Figure 3.
Structural analysis in retrieved allografts. Gross macroscopic images of representative (A-D) knee joints, and (E-L) individual retrieved FROZEN (blue), FRESH (green), 4°C/14d (orange), and 4°C/28d (purple) allografts at (E-H) MFC and (I-L) LT sites after 12months in vivo. Numbers in the lower right corner indicate the gross score assigned to (and components) are indicated below each representative image as surface+fill+integration=total.
Table 1.
Effect of in vivo allograft storage on gross- and histopathology-scores.
| MFC & LT | Non-OP | FROZEN | FRESH | 4°C/14d | 4°C/28d |
|---|---|---|---|---|---|
|
| |||||
| n | 15 | 7 | 7 | 8 | 8 |
| Gross a | 0.3 ± 0.1 *** | 7.4 ± 0.7 | 3.9 ± 0.7**,### | 3.3 ± 0.5***,### | 4.4 ± 0.7*,### |
| Surface Texture | 0.3 ± 0.1*** | 2.4 ± 0.3 | 1.1 ± 0.3**,### | 1.1 ± 0.2*,### | 1.5 ± 0.3### |
| Defect Filling/Size | 0.0 ± 0.0*** | 2.4 ± 0.4 | 1.0 ± 0.3*,### | 0.4 ± 0.2***,# | 0.8 ± 0.3*,### |
| Cartilage Integration | 0.0 ± 0.0*** | 2.6 ± 0.2 | 1.9 ± 0.2*,### | 1.8 ± 0.2**,### | 2.1 ± 0.2### |
|
| |||||
| Histopathology b | 3.4 ± 0.4 *** | 12.1 ± 0.7 | 4.4 ± 1.2 *** | 5.4 ± 0.4*,# | 6.1 ± 0.7*,## |
| Surface Irregularity | 0.7 ± 0.1** | 1.7 ± 0.1 | 0.6 ± 0.3** | 1.1 ± 0.2 | 1.3 ± 0.3 |
| Vertical Clefts | 0.4 ± 0.1* | 1.3 ± 0.3 | 0.5 ± 0.3 | 0.3 ± 0.1** | 0.5 ± 0.3 |
| Transverse Clefts | 0.4 ± 0.1* | 1.1 ± 0.3 | 0.3 ± 0.2 | 0.3 ± 0.1** | 0.4 ± 0.2 |
| Cloning | 0.1 ± 0.1*** | 2.0 ± 0.2 | 1.6 ± 0.3## | 1.3 ± 0.2## | 1.2 ± 0.2# |
| Hypocellularity | 0.3 ± 0.1*** | 3.0 ± 0.0 | 0.3 ± 0.1*** | 1.3 ± 0.2**,$$$,### | 1.2 ± 0.2**,$$$,### |
| Safranin-O | 1.1 ± 0.2*** | 3.0 ± 0.0 | 1.1 ± 0.3*** | 1.3 ± 0.3** | 1.6 ± 0.3* |
See Appendix Table 1 for scores at MFC and LT sites.
Gross-score (0-9) was determined by assigning a score of 0-3 (with 0 representing normal, and 3 representing severe degeneration) for the following gross morphological characteristics: surface texture, defect filling/size, and graft-recipient cartilage integration.13
Histopathology-score (0-15) was determined by assigning a score of 0-2 or 0-3 (with 0 representing normal, and 2 or 3 representing severe degeneration) for the following histologic characteristics: surface irregularity (0-2), vertical clefts into the transitional or radial zone (0-2), transverse clefts (0-2), cloning, (0-3), hypocellularity (0-3), and Safranin-O staining (0-3).38
p<0.05
p<0.01
p<0.001 vs. FROZEN
p<0.05
p<0.01
p<0.001 vs. Non-OP
p<0.05
p<0.01
p<0.001 vs. FRESH
Histopathology-score varied with allograft storage (p<0.001). Non-OP histopathology score was 3.4±0.4, similar to FRESH (p>0.9), and lower than FROZEN (p<0.001), 4°C/14d (p<0.05), and 4°C/28d (p<0.01, Table 1). Histopathology-score tended to increase with increasing 4°C storage duration. Histopathology-score was 4.4±1.2 in FRESH, and tended to be lower in 4°C/14d (p=0.2) and 4°C/28d (p=0.1) stored allografts. Histopathology-score was 12.1±0.7 in FROZEN, and was higher than FRESH (p<0.001), 4°C/14d (p<0.05), and 4°C/28d (p<0.05). Surface irregularity followed similar trends to overall histopathology-score, while vertical clefts, transverse clefts, and cloning were similar among FRESH, 4°C/14d, and 4°C/28d (p>0.7). Hypocellularity score in 4°C/14d and 4°C/28d stored allografts was higher than FRESH (each, p<0.001) and Non-OP (each, p<0.001), but lower than FROZEN (each, p<0.01, Table 1).
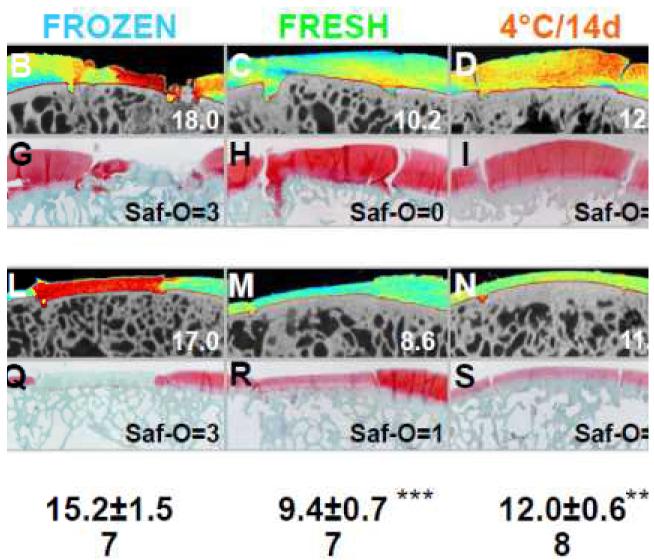
Quantitatively, in addition to visualizing overall qualitative patterns, HE-μCT varied with allograft storage (p<0.001). HE-μCT value in Non-OP was similar to FRESH (p>0.6, Fig. 4). Matrix fixed charge tended to be lower in 4°C/14d and 4°C/28d stored allografts than FRESH (p=0.1, p=0.2, respectively), as indicated by ~20% increase in HE-μCT value (95%CI: 12-31% for 4°C/14d and 9-30% for 4°C/28d, Fig. 4). Matrix fixed charge was lowest in FROZEN; HE-μCT value in FROZEN was ~35% higher than Non-OP and FRESH (95% CI: 21-49%; each, p<0.001), and ~22% higher than 4°C/14d and 4°C/28d stored allografts (95% CI: 10-35%; each, p<0.05).
Figure 4.
Analysis of matrix fixed charge in retrieved allografts. Representative (A-E,K-O) color maps of HE-μCT analysis and corresponding (F-J,P-T) Safranin-O histology of retrieved osteochondral allografts at (A-J) MFC and (K-T) LT sites after 12months in vivo. Values in lower right corner correspond to the HE-μCT value or Safranin-O score of the representative image. Mean±SEM. **p<0.01, ***p<0.001 vs. FROZEN.
Collectively, HE-μCT analysis and Safranin-O histology indicated qualitatively that matrix fixed charge was similar between Non-OP and FRESH, slightly decreased in 4°C/14d and 4°C/28d, and further decreased in FROZEN allografts. Highest HE-μCT values (in red), which represent low matrix fixed charge, were present throughout the depth of FROZEN (Fig. 4B,L). Moderate HE-μCT values (in orange and yellow) were present in 4°C/14d and 4°C/28d (Fig. 4D-E,N-O), and low HE-μCT values (in blue), which represent high matrix fixed charge, was absent in FROZEN, and present in Non-OP and FRESH (Fig. 4A,C,K,M). Representative Safranin-O histology confirmed the mild loss of proteoglycans in 4°C/14d and 4°C/28d, and extensive loss in FROZEN versus Non-OP and FRESH allografts (Fig. 4F-J,P-T). Safranin-O score in Non-OP was similar to FRESH (p>1.0, Table 1). Safranin-O staining intensity was consistently high in FRESH (Saf-O=0, 5/7 allografts), moderate in 4°C/14d (Saf-O=1, 6/8), and weak in 4°C/28d (Saf-O=2, 5/8). Safranin-O score was 3.0±0.0, indicating absence of staining, in FROZEN, and higher than all storage treatments (p<0.001 vs. Non-OP and FRESH, p<0.01 vs. 4°C/14d and 4°C/28d, Table 1).
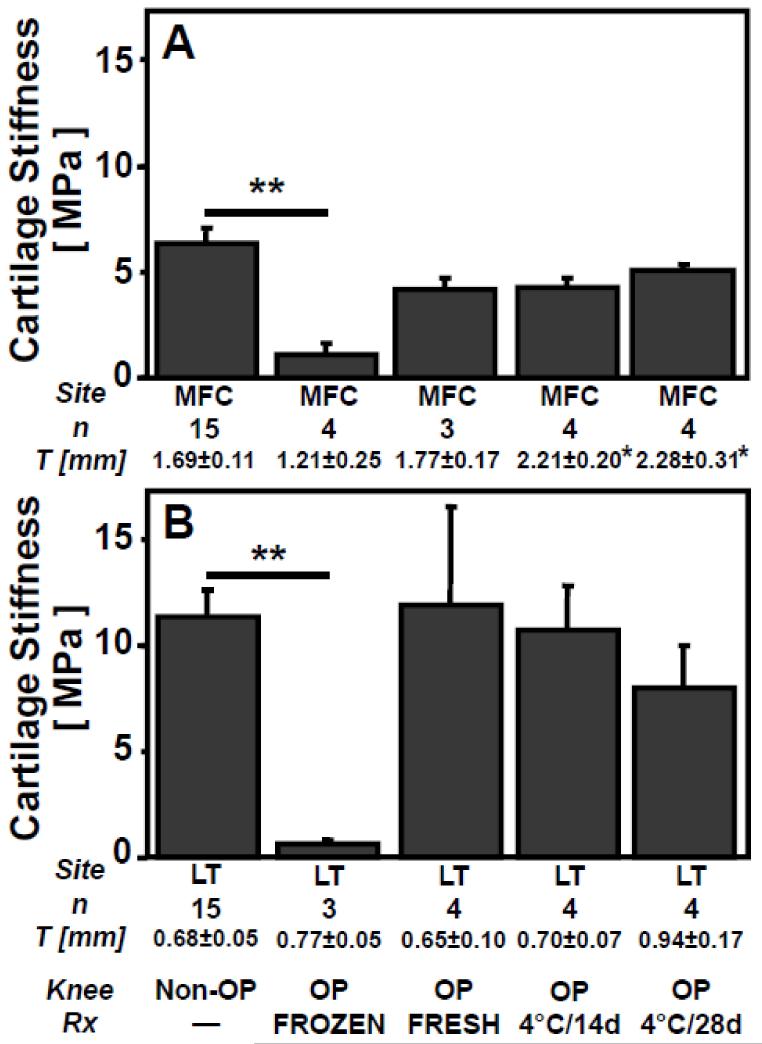
Cartilage material stiffness varied with allograft storage (p<0.001), and with site (p<0.001), but without an interaction effect (p=0.3). Stiffness in Non-OP was similar to FRESH, 4°C/14d, and 4°C/28d at MFC (p>0.4, Fig. 5A) and at LT (p>0.8, Fig. 5B). Stiffness in FROZEN was lower than Non-OP, by ~80% at MFC (95%CI: 72-91%; p<0.01) and by ~95% at LT (95%CI: 93-97%; p<0.01). Stiffness in FROZEN also tended to be lower than FRESH, 4°C/14d, and 4°C/28d, by ~75% at MFC (95%CI: 57-92%; p=0.3 vs. FRESH, p=0.2 vs. 4°C/14d, p=0.08 vs. 4°C/28d), and by ~94% at LT (95%CI: 88-99%; p=0.06 vs. FRESH, p=0.1 vs. 4°C/14d, p=0.4 vs. 4°C/28d).
Figure 5.
Effect of in vivo allograft storage on cartilage stiffness and thickness (T) in retrieved osteochondral allografts at (A) MFC and (B) LT sites after 12months. Mean±SEM. *p<0.05, **p<0.01 vs. FROZEN.
Cartilage thickness varied with allograft storage (p<0.01), site (p<0.001), and with an interaction effect (p<0.05). Cartilage thickness was ~40% thinner (95%CI: 36-45%) at LT (0.72±0.21mm) than at MFC (1.78±0.54mm, Fig. 5). At MFC, cartilage thickness in 4°C/14d and 4°C/28d was ~45% thicker (95%CI: 28-65%; p<0.05) than FROZEN, but similar to FRESH (p>0.6).
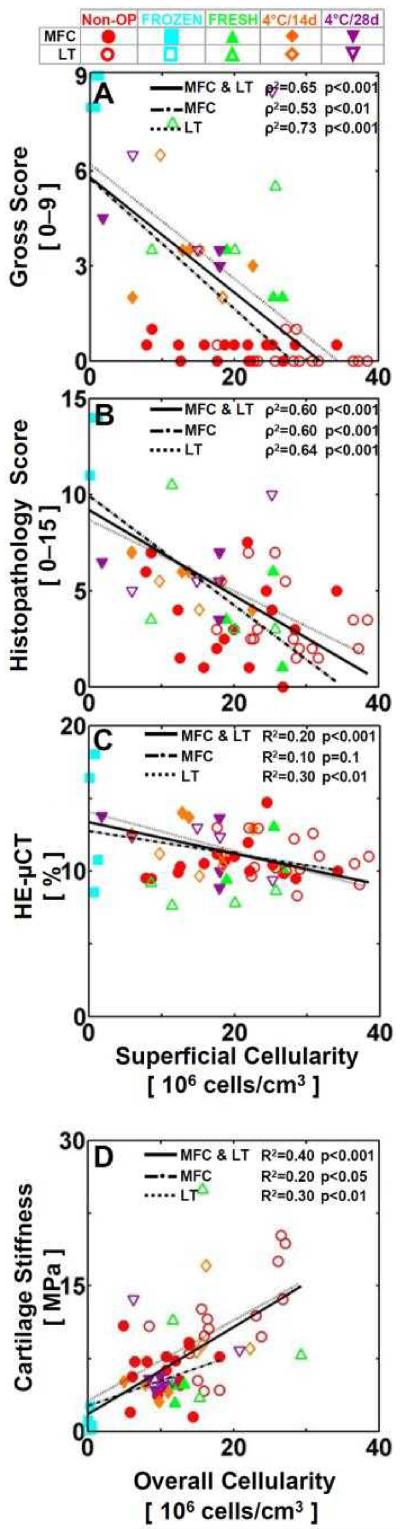
Indices of cartilage health, including gross-score, histopathology-score, matrix fixed charge, and cartilage stiffness, correlated significantly with cartilage cellularity (see Appendix Table 2). For MFC<, gross-score and histopathology-score correlated negatively with superficial cellularity (each, ρ2=0.6, p<0.001, Fig. 6A-B). HE-μCT value, inversely related to matrix fixed charge, also correlated negatively with superficial cellularity (R2=0.2, p<0.001, Fig. 6C), while cartilage stiffness correlated positively with overall cellularity (R2=0.4, p<0.01, Fig. 6D). MFC and LT also exhibited similar trends to MFC<, except HE-μCT value versus superficial cellularity for MFC (R2=0.1, p=0.1, Fig. 6C), and gross- and histopathology-score versus deep cellularity for MFC (ρ2=0.3, p=0.1, Fig. 6A,B).
Figure 6.
Relationship between cellularity and cartilage structure, composition, and function. (A,B) Spearman’s rank correlation and (C,D) parametric regression analysis of (A) gross score, (B) histopathology score, (C) HE-μCT value, and (D) cartilage stiffness vs. (A-C) superficial and (D) overall cellularity for MFC (filled) and LT (open). Data points correspond to individual Non-OP (red circles), FROZEN (blue squares), FRESH (green triangles), 4°C/14d (orange diamonds), and 4°C/28d (purple inverted triangles) allograft retrievals. Significance (p) and regression coefficients (ρ2 for A-B, R2 for C-D) were determined.
Discussion
The results demonstrate that reduced cellularity at the articular surface of osteochondral allografts resulting from various storage conditions was associated with decreased matrix content, stiffness, macroscopic structure, and histological outcomes, and that 4°C storage resulted in cartilage with reduced cellularity, matrix content, and structural scores compared to fresh allografts at 12months in the goat. The cartilage of FRESH osteochondral allografts, representing the best condition of implant viability, had similar tissue properties to Non-OP site-matched cartilage, and appeared smooth and flush to surrounding host cartilage (Fig. 3); chondrocytes were organized normally, decreasing with depth from the surface (Figs. 1-2), and provided sufficient biological remodeling capacity to sustain matrix content (Fig. 4) and mechanical properties (Fig. 5) of cartilage. In contrast, the cartilage of FROZEN osteochondral allografts, representing the worst condition of implant viability, was acellular (Fig. 1-2), and resulted in mechanically soft tissue (Fig. 5) that was structurally deteriorated (Fig. 3, Table 1) and depleted of matrix fixed charge/proteoglycans (Fig. 4). Intermediately, the cartilage of 4°C-stored osteochondral allografts was inferior to FRESH grafts, but marginally better than FROZEN grafts. Cellularity was reduced at the articular surface (Figs. 1-2), and accompanied by reduced matrix content (Fig. 4) and structural properties (Fig. 3, Table 1), suggesting that decreased cellularity, as a result of 4°C storage, detrimentally affects repair outcomes.
The associated decline in surface cellularity with overall tissue properties in retrieved osteochondral allografts suggests that 4°C-stored grafts are somewhat susceptible to tissue degeneration. Early signs of tissue structural deterioration in 4°C-stored grafts, including reduced proteoglycan content, increased surface irregularity, and poor graft-host cartilage integration, suggest inadequate biological remodeling capacity of the remaining chondrocytes to sustain long-term function. Although variable gross scores (range 1-9) and qualitatively diminished surface cellularity for 4°C-stored allografts were consistent with previous animal studies,21,34,37 the significant relationship between surface cellularity and repair outcomes reported here suggests that chondrocytes at the articular surface are vitally important to maintain tissue structure, composition, and function. Since surface chondrocyte viability during allograft storage appears to be a strong determinant of tissue properties, storage conditions that preserve surface viability is essential for long-term repair efficacy.
While reduced cellularity at the articular surface, as a result of 4°C storage, detrimentally affected repair efficacy in the goat, translation to the clinical situation may be dependent on several factors. Measuring cellularity within 100μm from the articular surface distinguished a region of cartilage in the goat vulnerable to cell death. In human cartilage, cells within an absolute distance from the surface may be affected, or this vulnerable region may scale relative to cartilage thickness. Although chondrocyte viability was similar between human31,32 and caprine22,26 in osteochondral tissue following clinically established 4°C storage conditions, in vivo repair efficacy in humans may be related to an absolute minimum number of cells rather than overall/regional cell density important in the goat model. Clinical repair outcomes may be more sensitive to 4°C storage duration, since human cartilage is ~3-5x less cellular than goat.16,32,46 Thus, evidence of reduced repair outcomes with increasing storage in this report suggests that 4°C storage duration should be limited prior to allograft implantation in patients.
Consistently good repair outcomes at MFC with FRESH allografts may be due to normal chondrocyte density in vivo, maintaining homeostasis of the osteochondral unit. Appropriate zonal chondrocyte organization in FRESH allografts provided biological remodeling to preserve the articular surface, sustain bulk tissue properties, and prevent progressive deterioration to graft failure (Figs. 1-5). Such maintenance of bulk tissue properties allows cartilage to function normally by supporting joint loads through pressurizing interstitial fluid,2 and preventing excessive tissue deformation and fluid exudation into the underlying subchondral bone plate, which would accelerate cartilage and bone degeneration. Variable results with FRESH allografts at LT may be due to inherent differences between the sites (i.e. cartilage thickness, loading environment), and are consistent with clinical results suggesting that patellofemoral lesions have worse clinical outcomes than femoral condyle lesions.4,9,18,41 Thus, repair outcomes for FRESH allografts serve as a good baseline from which to compare the outcomes of 4°C-stored grafts.
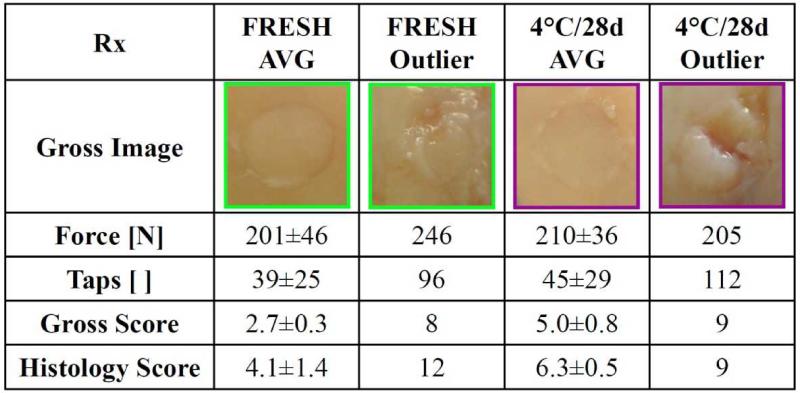
Osteochondral allograft insertion forces and impulses reported here were generally below the levels to cause significant cell death,19,30,39 and were consistent with previous human cadaveric6,30 and animal15 studies. While chondrocyte viability, especially at the articular surface, is generally inversely related to impact stress,10,19,30,39,42 the relationship between long-term performance and graft insertion parameters may be dependent of other factors as well. Repetitive trauma causes chondrocyte death, even if each impact is below the threshold for cartilage injury.8 In the present study, low gross and histological scores, in association with decreased surface cellularity, for two grafts, which required many more taps than average (~100 taps, Appendix Fig. 2), may suggest that tap number is also an important insertion parameter to consider. The decreased performance of these grafts suggests that preserving chondrocyte viability during insertion is also critically important to long-term repair efficacy.
Examining chondrocyte organization by depth following cartilage repair treatments and its association with repair efficacy may help elucidate a critical number of functional chondrocytes within the appropriate cartilage zone(s). Superficial zone cellularity, ≥18×106cells/cm3, was essential to maintain gross structural scores ≤3, which would characterize most FRESH allografts (5/7). Thus, most 4°C stored allografts, with ~15×106cells/cm3 in the superficial zone, did not have sufficient surface cellularity to maintain cartilage structure. In addition, the strong correlation of overall cellularity with cartilage function suggests the importance of maintaining cellularity throughout the depth of cartilage. However, since early tissue degeneration is evident at the articular surface, maintenance of superficial zone cellularity may be a crucial contributor to repair efficacy.
The inferior repair outcomes from 4°C-stored versus fresh allografts suggest that alternative storage protocols that preserve the chondrocyte viability, especially in the superficial zone are needed to improve long-term repair efficacy. Shorter storage duration (<7days) that more closely mimics the FRESH condition is one potential option. Alternatively, osteochondral allograft storage at 37°C may be one option to support long-term viability of chondrocyte in vitro, especially at the articular surface.5,22,26 Grafts stored at 37°C may also have better biological performance than 4°C-stored grafts, by providing lubricating molecules such as proteoglycan-4 (PRG4) to maintain a low-friction articulating surface.35,36 Since some 4°C-stored allografts maintained relatively high surface cellularity, an alternative biological marker of superficial zone health may be useful to screen grafts prior to implantation. PRG4-secreting function of allografts appears to be maintained in vivo based on its state after storage;28 thus, PRG4 secretion may be a useful predictor of suitable graft tissue. The long-term efficacy of allografts stored in conditions to enhance superficial zone chondrocyte viability (i.e. 37°C storage) remains to be elucidated.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR055637 (and -03S1) and AR044058) and an award to UCSD from the Howard Hughes Medical Institute through the HHMI Professors Program (for RLS). We thank Karen D. Bowden for technical histology assistance.
Appendix
Table A1.
Detailed Gross and Histopathology Scores at MFC and LT.
| MFC | Non-OP | FROZEN | FRESH | 4°C/14d | 4°C/28d |
|---|---|---|---|---|---|
| n | 15 | 4 | 3 | 4 | 4 |
| Gross | 0.4 ± 0.1*** | 8.5 ± 0.3 | 2.7 ± 0.3**,### | 3.5 ± 0.4*,### | 4.0 ± 0.6### |
| Surface Texture | 0.4 ± 0.1*** | 2.8 ± 0.1 | 0.7 ± 0.2** | 1.1 ± 0.1*** | 1.3 ± 0.1* |
| Defect Filling/Size | 0.0 ± 0.0*** | 2.8 ± 0.1 | 0.5 ± 0.3*** | 0.3 ± 0.1*** | 0.4 ± 0.1*** |
| Cartilage Integration | 0.0 ± 0.0*** | 3.0 ± 0.0 | 0.3 ± 0.3*** | 1.6 ± 0.2$$,### | 1.9 ± 0.2$$,### |
| Histopathology | 3.5 ± 0.6*** | 13.3 ± 0.8 | 3.5 ± 1.4** | 5.8 ± 0.6$,# | 6.5 ± 1.2$,# |
| Surface Irregularity | 1.0 ± 0.2 | 1.8 ± 0.3 | 0.8 ± 0.6 | 1.3± 0.3 | 1.0 ± 0.4 |
| Vertical Clefts | 0.6 ± 0.1* | 1.8 ± 0.3 | 0.3 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.5* |
| Transverse Clefts | 0.5 ± 0.1** | 1.4 ± 0.4 | 0.3 ± 0.3* | 0.4 ± 0.1* | 0.3 ± 0.3 |
| Cloning | 0.2 ± 0.1*** | 2.4 ± 0.2 | 1.2 ± 0.3*,### | 0.9 ± 0.2***,# | 1.5 ± 0.4%,# |
| Hypocellularity | 0.4 ± 0.1*** | 3.0 ± 0.0 | 0.3 ± 0.2*** | 1.6 ± 0.3$$,## | 1.1 ± 0.3*,$,# |
| Safranin-O | 0.7 ± 0.2** | 3.0 ± 0.0 | 0.5 ± 0.3* | 1.3 ± 0.5 | 2.1 ± 0.3$ |
| LT | Non-OP | FROZEN | FRESH | 4°C/14d | 4°C/28d |
|---|---|---|---|---|---|
| n | 15 | 3 | 4 | 4 | 4 |
| Gross | 0.2 ± 0.1*** | 5.8 ± 1.2 | 5.0 ± 1.0### | 3.5 ± 1.1### | 5.4 ± 1.3### |
| Surface Texture | 0.2 ± 0.1** | 1.8 ± 0.4 | 1.4 ± 0.4# | 1.1 ± 0.4 | 1.8 ± 0.6# |
| Defect Filling/Size | 0.0 ± 0.0*** | 1.8 ± 0.6 | 1.4 ± 0.4### | 0.5 ± 0.4**,$ | 1.3 ± 0.5%%,### |
| Cartilage Integration | 0.0 ± 0.0*** | 2.2 ± 0.3 | 2.3 ± 0.3### | 2.0 ± 0.4### | 2.4 ± 0.2### |
| Histopathology | 3.5 ± 0.6*** | 10.7 ± 0.9 | 5.0 ± 1.8 | 5.1 ± 0.4# | 6.5 ± 1.2# |
| Surface Irregularity | 0.4 ± 0.2 | 1.7 ± 0.2 | 0.4 ± 0.4 | 0.9 ± 0.2 | 1.0 ± 0.4 |
| Vertical Clefts | 0.2 ± 0.1 | 0.7 ± 0.3 | 0.6 ± 0.5 | 0.0 ± 0.0 | 0.5 ± 0.5 |
| Transverse Clefts | 0.2 ± 0.1 | 0.8 ± 0.6 | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.3 ± 0.3 |
| Cloning | 0.1 ± 0.1* | 1.5 ± 0.3 | 2.0 ± 0.4### | 1.8 ± 0.1### | 1.5 ± 0.4# |
| Hypocellularity | 0.1 ± 0.1*** | 3.0 ± 0.0 | 0.3 ± 0.3*** | 0.9 ± 0.3$,## | 1.1 ± 0.3$,## |
| Safranin-O | 1.5 ± 0.2** | 3.0 ± 0.0 | 1.5 ± 0.2 | 1.5 ± 0.8 | 2.3 ± 1.2 |
mean ± SEM, * vs. FROZEN, # vs. Non-OP, $ vs. FRESH, % vs. 4°C/14d
Table A2.
Regression analysis of indices of cartilage health versus cartilage cellularity.
| MFC & LT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellularity [ 106 cells/cm3 ] |
Gross | Histopathology | HE-μCT [ % ] | Stiffness [ MPa ] | ||||||||
| ρ2 | p< | m | ρ2 | p< | m | ρ2 | p< | m | ρ2 | p< | m | |
| Superficial | 0.65 | 0.001 | −0.18 | 0.60 | 0.001 | −0.22 | 0.20 | 0.001 | −0.11 | 0.16 | 0.01 | 0.20 |
| Middle | 0.41 | 0.01 | −0.13 | 0.48 | 0.001 | −0.23 | 0.14 | 0.01 | −0.11 | 0.37 | 0.001 | 0.36 |
| Deep | 0.39 | 0.01 | −0.12 | 0.47 | 0.001 | −0.20 | 0.09 | 0.05 | −0.10 | 0.38 | 0.001 | 0.44 |
| Overall | 0.49 | 0.001 | −0.18 | 0.52 | 0.001 | −0.27 | 0.15 | 0.01 | −0.13 | 0.40 | 0.001 | 0.45 |
| MFC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellularity [ 106 cells/cm3 ] |
Gross | Histopathology | HE-μCT [ % ] | Stiffness [ MPa ] | ||||||||
| ρ2 | p< | m | ρ2 | p< | m | ρ2 | p< | m | ρ2 | p< | m | |
| Superficial | 0.53 | 0.01 | −0.21 | 0.60 | 0.001 | −0.28 | 0.10 | 0.08 | 0.16 | 0.01 | 0.11 | |
| Middle | 0.45 | 0.05 | −0.34 | 0.66 | 0.001 | −0.56 | 0.06 | 0.20 | 0.37 | 0.001 | 0.20 | |
| Deep | 0.41 | 0.05 | −0.47 | 0.58 | 0.001 | −0.73 | 0.05 | 0.25 | 0.38 | 0.001 | 0.33 | |
| Overall | 0.52 | 0.01 | −0.44 | 0.67 | 0.001 | −0.66 | 0.08 | 0.13 | 0.20 | 0.001 | 0.26 | |
| LT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellularity [ 106 cells/cm3 ] |
Gross | Histopathology | HE-μCT [ % ] | Stiffness [ MPa ] | ||||||||
| ρ2 | p< | m | ρ2 | p< | m | ρ2 | p< | m | ρ2 | p< | m | |
| Superficial | 0.73 | 0.001 | −0.18 | 0.64 | 0.001 | −0.18 | 0.30 | 0.01 | −0.14 | 0.12 | 0.06 | |
| Middle | 0.44 | 0.05 | −0.13 | 0.45 | 0.05 | −0.20 | 0.26 | 0.01 | −0.15 | 0.26 | 0.01 | 0.34 |
| Deep | 0.29 | 0.11 | 0.31 | 0.10 | 0.14 | 0.05 | −0.12 | 0.27 | 0.01 | 0.37 | ||
| Overall | 0.54 | 0.01 | −0.17 | 0.47 | 0.01 | −0.23 | 0.26 | 0.01 | −0.17 | 0.30 | 0.01 | 0.42 |
Spearman rank (ρ2) and linear (R2) regression coefficients; significance (p); slope (m).
Figure A1.
Effect of in vivo allograft storage on cellularity by depth from the articular surface (0-0.6mm) in retrieved osteochondral allografts at (A) MFC and (B) LT sites after 12months. Mean±SEM, n=3-15. *p<0.05, **p<0.01, ***p<0.001 vs. FROZEN. # vs. Non-OP, $ vs. FRESH.
Figure A2.
Effect of in vivo allograft insertion for average and outlying samples on structural scores after 12months. Mean±SD.
References
- 1.Guidance for Industry: Screening and testing of human tissue intended for transplantation. USDoHaH: Food and Drug Administration Center for Biologics Evaluation and Research; Washington, DC: 2002. [Google Scholar]
- 2.Ateshian GA, Lai WM, Zhu WB, Mow VC. An asymptotic solution for the contact of two biphasic cartilage layers. J Biomech. 1994;27:1347–1360. doi: 10.1016/0021-9290(94)90044-2. PMID: 7798285. [DOI] [PubMed] [Google Scholar]
- 3.Ball ST, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL, Bugbee WD. The effects of storage media on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418:246–252. doi: 10.1097/00003086-200401000-00043. PMID: 15043126. [DOI] [PubMed] [Google Scholar]
- 4.Beaver RJ, Mahomed M, Backstein D, Davis A, Zukor DJ, Gross AE. Fresh osteochondral allografts for post-traumatic defects in the knee. J Bone Joint Surg Br. 1992;74:105–110. doi: 10.1302/0301-620X.74B1.1732235. PMID: 1732235. [DOI] [PubMed] [Google Scholar]
- 5.Bian L, Stoker AM, Marberry KM, Ateshian GA, Cook JL, Hung CT. Effects of dexamethasone on the functional properties of cartilage explants during long-term culture. Am J Sports Med. 2010;38:78–85. doi: 10.1177/0363546509354197. PMID: 19959744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borazjani BH, Chen AC, Bae WC, Patil S, Sah RL, Firestein GS, Bugbee WD. The effect of impact on chondrocyte viability during the insertion of human osteochondral grafts. J Bone Joint Surg. 2006;88:1934–1943. doi: 10.2106/JBJS.E.00992. PMID: 16951108. [DOI] [PubMed] [Google Scholar]
- 7.Bugbee WD. Fresh osteochondral allografts. J Knee Surg. 2002;15:191–195. PMID: 12152982. [PubMed] [Google Scholar]
- 8.Chen CT, Burton-Wurster N, Borden C, Hueffer K, Bloom SE, Lust G. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res. 2001;19:703–711. doi: 10.1016/S0736-0266(00)00066-8. PMID: 11518282. [DOI] [PubMed] [Google Scholar]
- 9.Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation: clinical results in the knee. Clin Orthop Relat Res. 1999;360:159–168. PMID: 10101321. [PubMed] [Google Scholar]
- 10.D’Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr., Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712–719. doi: 10.1053/joca.2001.0468. PMID: 11795990. [DOI] [PubMed] [Google Scholar]
- 11.Davidson PA, Rivenburgh DW, Dawson PE, Rozin R. Clinical, histologic, and radiographic outcomes of distal femoral resurfacing with hypothermically stored osteoarticular allografts. Am J Sports Med. 2007;35:1082–1090. doi: 10.1177/0363546507299529. PMID: 17351122. [DOI] [PubMed] [Google Scholar]
- 12.Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907–914. doi: 10.1177/0363546507299932. PMID: 17369560. [DOI] [PubMed] [Google Scholar]
- 13.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–288. doi: 10.1302/0301-620x.84b2.11167. PMID: 11922373. [DOI] [PubMed] [Google Scholar]
- 14.Glantz SA. Primer of Biostatistics. 3rd ed. McGraw-Hill, Inc.; San Francisco, CA: 1992. [Google Scholar]
- 15.Gulotta LV, Rudzki JR, Kovacevic D, Chen CC, Milentijevic D, Williams RJ., 3rd Chondrocyte death and cartilage degradation after autologous osteochondral transplantation surgery in a rabbit model. Am J Sports Med. 2009;37:1324–1333. doi: 10.1177/0363546509333476. PMID: 19448050. [DOI] [PubMed] [Google Scholar]
- 16.Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564–572. doi: 10.1053/joca.2002.0814. PMID: 12127837. [DOI] [PubMed] [Google Scholar]
- 17.Jadin KD, Wong BL, Bae WC, Li KW, Williamson AK, Schumacher BL, Price JH, Sah RL. Depth-varying density and organization of chondrocyte in immature and mature bovine articular cartilage assessed by 3-D imaging and analysis. J Histochem Cytochem. 2005;53:1109–1119. doi: 10.1369/jhc.4A6511.2005. PMID: 15879579. [DOI] [PubMed] [Google Scholar]
- 18.Jamali AA, Emmerson BC, Chung C, Convery FR, Bugbee WD. Fresh osteochondral allografts: results in the patellofemoral joint. Clin Orthop Relat Res. 2005;437:176–185. PMID: 16056047. [PubMed] [Google Scholar]
- 19.Kang RW, Friel NA, Williams JM, Cole BJ, Wimmer MA. Effect of impaction sequence on osteochondral graft damage: the role of repeated and varying loads. Am J Sports Med. 2010;38:105–113. doi: 10.1177/0363546509349038. PMID: 19915099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91:805–811. doi: 10.2106/JBJS.H.00703. PMID: 19339564. [DOI] [PubMed] [Google Scholar]
- 21.Malinin T, Temple HT, Buck BE. Transplantation of osteochondral allografts after cold storage. J Bone Joint Surg Am. 2006;88:762–770. doi: 10.2106/JBJS.D.02991. PMID: 16595466. [DOI] [PubMed] [Google Scholar]
- 22.McCarty WJ, Pallante AL, Rone RJ, Bugbee WD, Sah RL. The proteoglycan metabolism of articular cartilage in joint-scale culture. Tissue Eng Part A. 2010;16:1717–1727. doi: 10.1089/ten.tea.2009.0663. PMID: 20038199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35:411–420. doi: 10.1177/0363546506295178. PMID: 17261573. [DOI] [PubMed] [Google Scholar]
- 24.McDermott AG, Langer F, Pritzker KP, Gross AE. Fresh small-fragment osteochondral allografts. Long-term follow-up study on first 100 cases. Clin Orthop Relat Res. 1985;197:96–102. PMID: 3893835. [PubMed] [Google Scholar]
- 25.Oates KM, Chen AC, Young EP, Kwan MK, Amiel D, Convery FR. Effect of tissue culture storage on the in vivo survival of canine osteochondral allografts. J Orthop Res. 1995;13:562–569. doi: 10.1002/jor.1100130411. PMID: 7674072. [DOI] [PubMed] [Google Scholar]
- 26.Pallante AL, Bae WC, Chen AC, Gortz S, Bugbee WD, Sah RL. Chondrocyte viability is higher after prolonged storage at 37 degrees C than at 4 degrees C for osteochondral grafts. Am J Sports Med. 2009;37:24S–32S. doi: 10.1177/0363546509351496. PMID: 19861697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallante AL, Gortz S, Chen AC, Healey RM, Chase DC, Ball ST, Amiel D, Sah RL, Bugbee WD. Treatment of Articular Cartilage Defects in the Goat with Frozen versus Fresh Osteochondral Allografts: Effects on Cartilage Stiffness, Zonal Composition, and Structure at Six Months. J Bone Joint Surg. 2011 doi: 10.2106/JBJS.K.00439. in press. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pallante AL, Gortz S, Chen AC, Schumacher BL, Temple-Wong MM, Sah RL, Bugbee WD. Association of osteochondral allograft efficacy with PRG4 secretion in vivo in the goat; Paper presented at: Trans Orthop Res Soc; Long Beach, CA. 2011. [Google Scholar]
- 29.Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc Natl Acad Sci U S A. 2006;103:19255–19260. doi: 10.1073/pnas.0606406103. PMID: 17158799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patil S, Butcher W, D’Lima DD, Steklov N, Bugbee WD, Hoenecke HR. Effect of osteochondral graft insertion forces on chondrocyte viability. Am J Sports Med. 2008;36:1726–1732. doi: 10.1177/0363546508316765. PMID: 18490471. [DOI] [PubMed] [Google Scholar]
- 31.Pearsall AW, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125–131. doi: 10.1177/0095399703258614. PMID: 14754735. [DOI] [PubMed] [Google Scholar]
- 32.Pennock AT, Wagner F, Robertson CM, Harwood FL, Bugbee WD, Amiel D. Prolonged storage of osteochondral allografts: does the addition of fetal bovine serum improve chondrocyte viability? J Knee Surg. 2006;19:265–272. doi: 10.1055/s-0030-1248117. PMID: 17080649. [DOI] [PubMed] [Google Scholar]
- 33.Pylawka TK, Wimmer M, Cole BJ, Virdi AS, Williams JM. Impaction affects cell viability in osteochondral tissues during transplantation. J Knee Surg. 2007;20:105–110. doi: 10.1055/s-0030-1248028. PMID: 17486901. [DOI] [PubMed] [Google Scholar]
- 34.Ranawat AS, Vidal AF, Chen CT, Zelken JA, Turner AS, Williams RJ., 3rd Material properties of fresh cold-stored allografts for osteochondral defects at 1 year. Clin Orthop Relat Res. 2008;466:1826–1836. doi: 10.1007/s11999-008-0311-7. PMID: 18528743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. PMID: 17328061. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–120. doi: 10.1002/jor.1100170117. PMID: 10073655. [DOI] [PubMed] [Google Scholar]
- 37.Shahgaldi BF, Amis AA, Heatley FW, McDowell J, Bentley G. Repair of cartilage lesions using biological implants. J Bone Joint Surg Br. 1991;73-B:57–64. doi: 10.1302/0301-620X.73B1.1991776. PMID: 1991776. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro F, Glimcher MJ. Induction of osteoarthrosis in the rabbit knee joint. Histologic changes following menisectomy and meniscal lesions. Clin Orthop Relat Res. 1980;147:287–295. PMID: 6154558. [PubMed] [Google Scholar]
- 39.Szczodry M, Coyle CH, Kramer SJ, Smolinski P, Chu CR. Progressive chondrocyte death after impact injury indicates a need for chondroprotective therapy. Am J Sports Med. 2009;37:2318–2322. doi: 10.1177/0363546509348840. PMID: 19864505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng MS, Yuen AS, Kim HT. Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res. 2008;466:1804–1809. doi: 10.1007/s11999-008-0302-8. PMID: 18506560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torga Spak R, Teitge RA. Fresh osteochondral allografts for patellofemoral arthritis: long-term follow-up. Clin Orthop Relat Res. 2006;444:193–200. doi: 10.1097/01.blo.0000201152.98830.ed. PMID: 16523140. [DOI] [PubMed] [Google Scholar]
- 42.Torzilli PA, Grigiene R, Borrelli J, Jr., Helfet DL. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J Biomech Eng. 1999;121:433–441. doi: 10.1115/1.2835070. PMID: 10529909. [DOI] [PubMed] [Google Scholar]
- 43.Williams JM, Virdi AS, Pylawka TK, Edwards RB, 3rd, Markel MD, Cole BJ. Prolonged-fresh preservation of intact whole canine femoral condyles for the potential use as osteochondral allografts. J Orthop Res. 2005;23:831–837. doi: 10.1016/j.orthres.2004.07.007. PMID: 16022997. [DOI] [PubMed] [Google Scholar]
- 44.Williams RJ, 3rd, Dreese JC, Chen CT. Chondrocyte survival and material properties of hypothermically stored cartilage: an evaluation of tissue used for osteochondral allograft transplantation. Am J Sports Med. 2004;32:132–139. doi: 10.1177/0095399703258733. PMID: 14754736. [DOI] [PubMed] [Google Scholar]
- 45.Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718–726. doi: 10.2106/JBJS.F.00625. PMID: 17403792. [DOI] [PubMed] [Google Scholar]
- 46.Williams SK, Amiel D, Ball ST, Allen RT, Tontz WL, Jr, Emmerson BC, Badlani NM, Emery SC, Haghighi P, Bugbee WD. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35:2022–2032. doi: 10.1177/0363546507305017. PMID: 17724095. [DOI] [PubMed] [Google Scholar]
- 47.Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A:2111–2120. doi: 10.2106/00004623-200311000-00008. PMID: 14630839. [DOI] [PubMed] [Google Scholar]