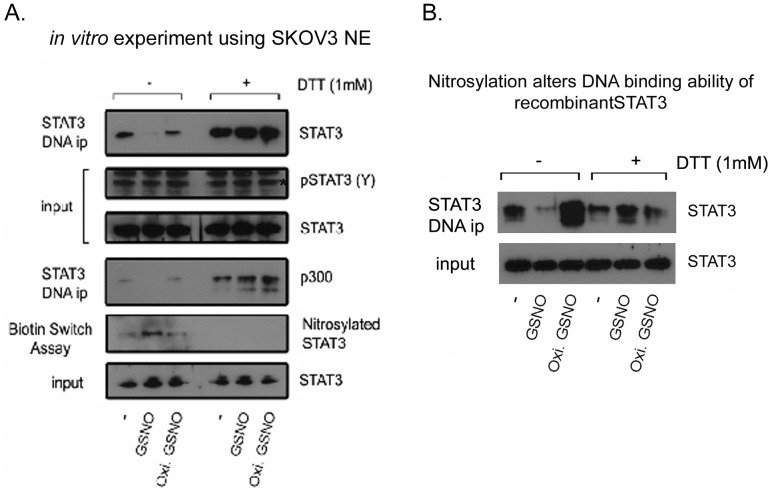
Figure 8. In vitro nitrosylation of STAT3 affects its DNA binding ability.
A. Nuclear extract (NE) from SKOV3 was isolated and 20 µg was incubated with S-nitrosoglutathione (GSNO) or oxidized GSNO (100 µM) at 4°C in the presence or absence of DTT (1 mM). After 4 hours of incubation, DNA binding ability of nuclear STAT3 was examined by incubating nitrosylated NE with STAT3 gel shift oligonucleotides conjugated with agarose followed by immunoblot analysis with anti-STAT3 antibody. Since STAT3 recruits coactivator, including p300, we also examined the recruitment of p300 under similar experimental conditions. STAT3 and pSTAT3 (Y705) were examined before pulling down STAT3 with gel shift oligonucleotides conjugated with agarose. Nitrosylation of STAT3 was also examined under similar experimental conditions using biotin switch method. Total STAT3 level was examined in the input of NE from SKOV3, which was used to show that an equal protein amount was used in this experiment. B. Recombinant STAT3 (1 µg) was incubated with GSNO or oxidized GSNO (0.1 mM) at 4°C in the presence or absence of DTT (1 mM). After 2 hours of incubation, DNA binding ability of recombinant STAT3 is examined by pulling down Stat3 by incubating with gel shift oligonucleotides conjugated with agarose.