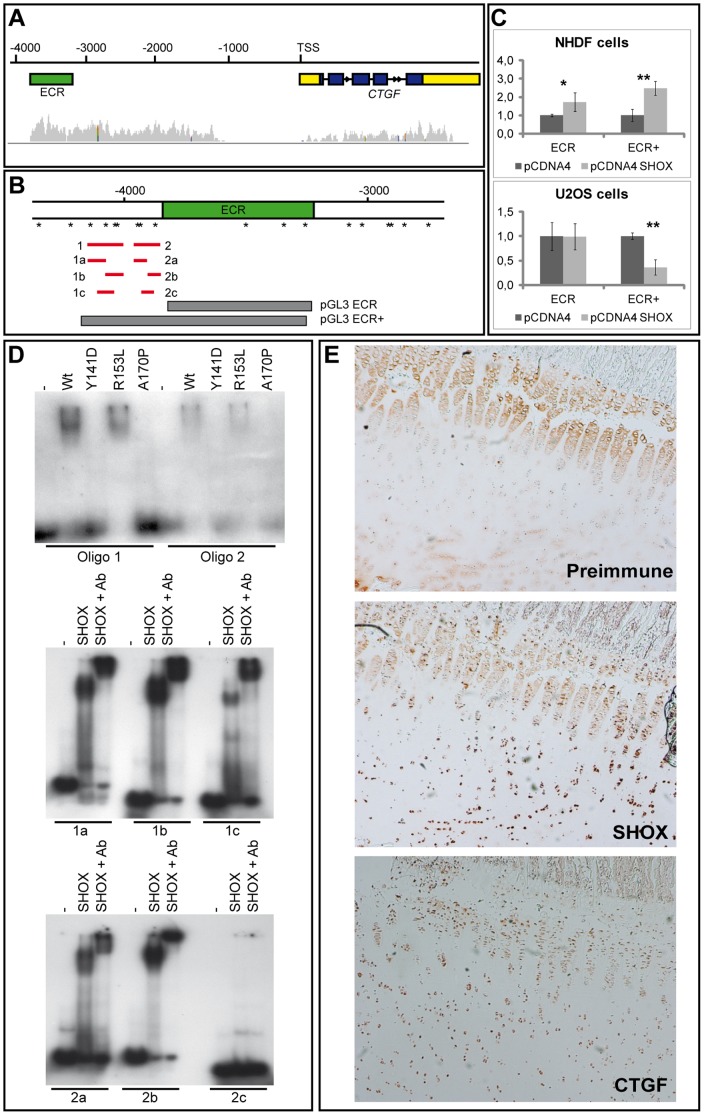
Figure 4. Analysis of CTGF as a direct transcriptional target of SHOX.
(A): Genomic structure of the human CTGF region. ChIP-Seq analysis in ChMM cultures revealed an accumulation of Shox binding in the Ctgf promoter region (grey peaks), especially in a region 3–4 kb from the transcriptional start site (TSS) where an evolutionary conserved sequence (ECR) of 597 bp (human chr6:132317086-132318077) was identified (green bar). (B): Location of the pGL3 ECR and pGL3 ECR+ reporter constructs (grey bars) within the CTGF upstream region. The ECR+ construct encompasses the ECR and an upstream region including ATTA/TAAT motifs and palindromes. SHOX binding motifs (ATTA/TAAT sites and palindromes) in the CTGF 5′ region around the ECR are indicated by asterisks. Red bars represent the location of the generated oligonucleotides for EMSA. (C): Luciferase reporter gene assays in NHDF and U2OS cells. pcDNA4/TO SHOX was cotransfected with a luciferase reporter vector harbouring either the ECR or the ECR+ sequence. Transfections and measurements were carried out in triplicates. A significant activation in the luciferase activity was observed 24 h after SHOX transfection in NHDF cells using both reporter constructs (1.7-fold/2.5-fold with p = 0.02/0.007 for ECR/ECR+). In U2OS cells, an alteration was not observed for the ECR reporter, but a significant reduction was demonstrated for the ECR+ reporter construct (1.0-fold/2.8-fold with p = 0.1/0.003 for ECR/ECR+). (D): EMSA. The SHOX wildtype (Wt) and the mutant p.R153L proteins bind to oligonucleotides 1 and 2, whereas the defective proteins p.Y141D and p.A170P cannot. All fragments of oligonucleotides 1 and 2 containing an ATTA/TAAT site are sensitive to SHOX binding (1a–c, 2a–b). The fragment lacking this motif does not bind (oligonucleotide 2c). Using the SHOX-3 antibody (Ab), we demonstrate that the binding is SHOX-specific. (E): Immunohistochemistry performed on pubertal tibial growth plates. Staining was performed using preimmune serum as a negative control, SHOX antibody [19] and a CTGF-specific antibody. Both the SHOX and CTGF proteins were detected in growth plate chondrocytes.