Figure 1.
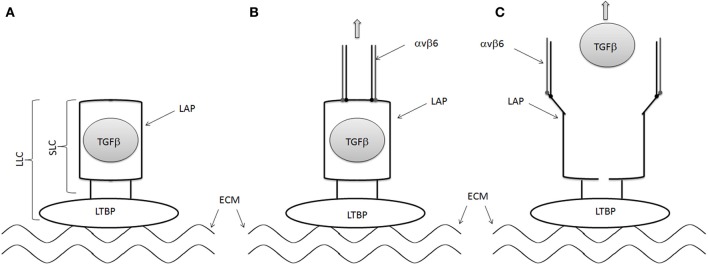
Mechanisms of TGFβ activation by αvβ6 integrin. (A) LAP forms a straightjacket around TGFβ in the small latent complex (SLC) and binds to LTBP to for the large latent complex (LLC). The LTBP is in turn tethered to the extracellular matrix. (B) The cell associated αvβ6 integrin binds to the RGD domains of LAP, pulling the LAP ope against the resistance provided by the extracellular matrix/LTBP interaction. (C) TGFβ is then released from the LAP, allowing it to interact with its receptor.
