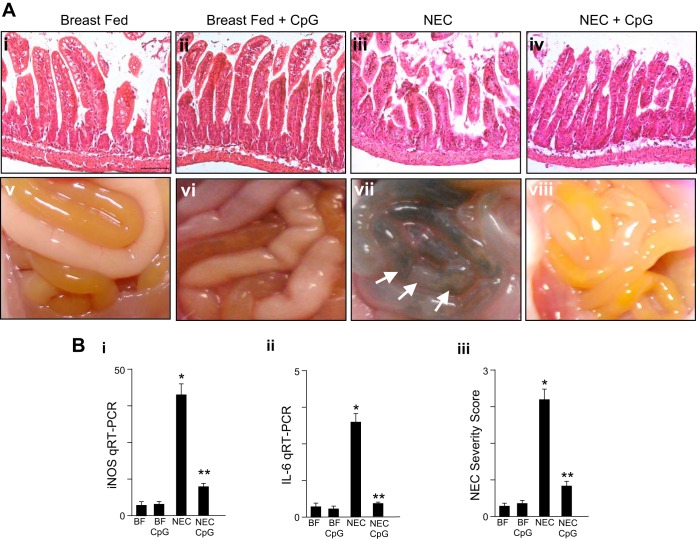
Fig. 3.
The oral administration of oral CpG-DNA ODN-1826 attenuates NEC severity in mice. A: representative photomicrographs of the terminal ileum (i–iv) and gross images of the intestines (v–viii) from neonatal wild-type mice that were either BF controls or induced to develop NEC in the presence or absence of oral CpG-DNA (ODN-1826 1 mg/kg) once daily. B: NEC severity as assessed by the terminal ileal expression of iNOS by qRT-PCR (i), IL-6 mRNA expression by qRT-PCR (ii), or NEC severity score as determined by a pathologist blinded to the identity of the groups (iii). In all graphs, *P < 0.05 NEC vs. BF control; **P < 0.05 NEC vs. NEC in mice treated with oral CpG-DNA. Data are means ± SE. Size bar = 50 μm.