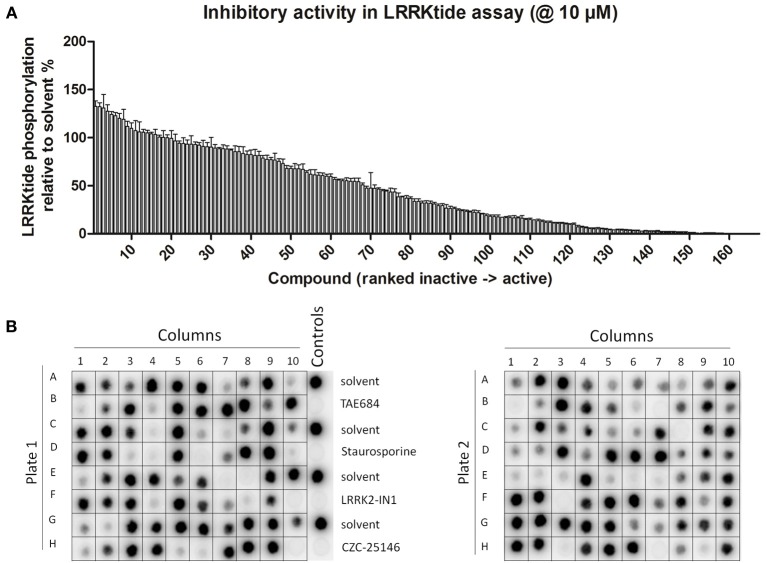
Figure 1.
Testing of effect of reference kinase inhibitors on in vitro LRRK2 kinase activity. (A,B) 160 kinase inhibitors from a panel of inhibitors known to target kinases in all branches of the kinome were tested for their ability to inhibit LRRK2 at 10 μM in an in vitro kinase assay using the LRRKtide model peptide substrate, as described in Materials and Methods. (A) Quantification of the LRRKtide phosphorylation level for each kinase reaction. Signal intensity per reaction was quantified via densitometry as described in Materials and Methods and values are normalized to phosphorylation levels measured in solvent controls (control values are set at 100%). Values obtained (mean ± s.e.m., N = 3) are depicted as histogram bars ordered from least active to most active compound, showing that the panel comprises a broad range of activity on LRRK2 kinase function. Exact values are given in Table 1. (B) Representative autoradiograms of P81 paper spotted with kinase reactions from testing of the 160 compounds which were used for densitometric quantification given in A. Also shown at the right of the first panel are the solvent controls as well as positive controls using potent inhibitors of the LRRK2 kinase, TAE684, staurosporine, LRRK2-IN1, and CZC-25146. Illustration of detailed IC50 determination for active compounds is given in Figure 2.