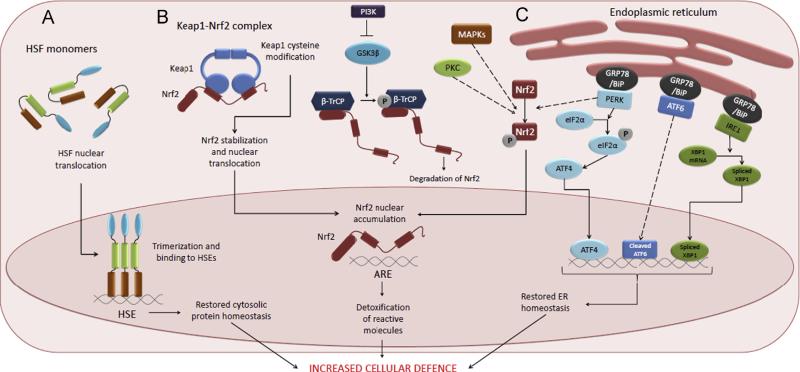
Fig. 1.
Stress signaling pathways activated by electrophiles. (A) Heat shock response (HSR). Upon activation, heat shock factors (HSFs), primarily HSF1, translocate to the nucleus, trimerize and bind to heat shock elements (HSEs) and drive the expression of target genes, such as heat shock proteins. (B) Keap1-Nrf2 pathway. During electrophile stress, Keap1 is no longer able to deliver Nrf2 to proteasomal degradation, and the stabilized Nrf2 protein translocates to the nucleus, binds to antioxidant response elements (AREs), and regulates target genes. Keap1-independent mechanisms for Nrf2 nuclear accumulation include phosphorylation of Nrf2 by PKC, PERK, or MAP Kinases. In addition, PI3K may regulate Nrf2 via indirect mechanism by inhibiting GSK-3β activity. Active GSK-3β phosphorylates Nrf2 Neh6 domain binding protein β-TrCP, which results in Cul1-dependent ubiquitination and degradation of Nrf2. (C) Unfolded protein response (UPR). Accumulation of misfolded proteins in the ER activates the three arms of the UPR, resulting in activation of three transcription factors that regulate the expression of UPR target genes. PERK kinase can also phosphorylate and activate Nrf2.