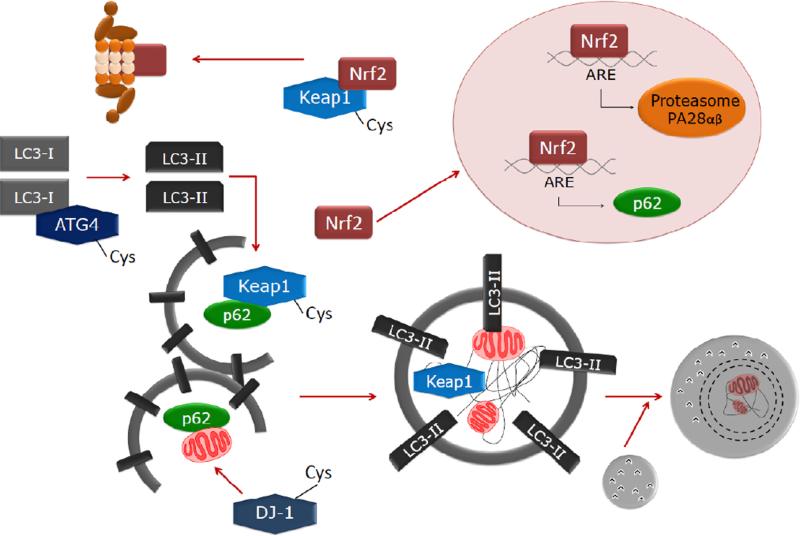
Fig. 2.
Redox regulation of autophagy. The Keap1-Nrf2 pathway is not only regulated by the proteasomes but also by autophagic activity. The cysteine-rich protein Keap1 serves as a redox sensor and can bind to p62 and be degraded by autophagic activity. Undegraded Keap1 complexes with Nrf2 and mediates Nrf2 ubiquitination and degradation by the proteasomes. In response to oxidants, cysteine modification on Keap1 releases Nrf2 by inhibiting its ubiquitination. Keap1-free Nrf2 is stabilized and transported to the nucleus where it activates transcription of antioxidant genes, including HO-1 and NQO1, proteasomal PA28αβ, thereby increases proteasomal degradation of oxidatively damaged proteins as well as p62, which plays an important role in autophagy by interacting with both ubiquitinated proteins and microtubule-associated protein light chain 3 (LC3), thereby facilitates autophagy-lysosomal degradation of proteins and dysfunctional organelles such as the mitochondria. Some of the autophagy proteins are also susceptible to cysteine modification, examples include ATG4 which is a cysteine protease involved in pro-LC3 cleavage, LC3I to LC3II conversion by lipidation, and LC3II to LC3I conversion by delipidation. DJ-1 is a cysteine protease playing a role in mitochondrial quality control and DJ-1 mutant mice or human Parkinson's disease patients accumulate abnormal mitochondria.