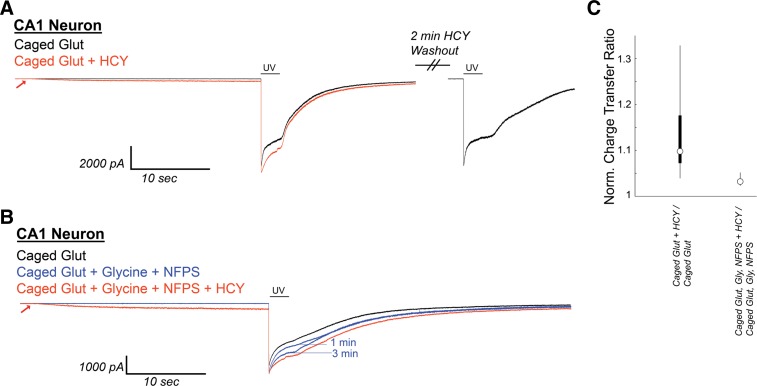
Fig. 12.
HCY induces a small initial depolarization and reduces GLY-dependent NMDAR desensitization in neurons from acute CA1 slices during uncaging of 1 mM MNI-caged glutamate. A: CA1 neurons in acute hippocampal slices [N = 6 from postnatal day 10 (P10) to P17 mice, N = 6 from P43–P58 mice] were voltage clamped in the presence of 300 nM tetrodotoxin, 10 μM NBQX, and 20 μM gabazine. Under these conditions, Schaffer collateral axon stimulation (200 μs) consistently evoked currents at +40 mV, but not at −70 mV holding, indicating the isolation of NMDAR currents. After Mg-washout, MNI-caged glutamate (1 mM) was perfused onto the neuron, and a 2-s flash of UV light was used to uncage glutamate. This induced an NMDAR current with pronounced desensitization. If HCY (1 mM d,l-HCY = 500 μM l-HCY) was washed onto the slice 30s before UV uncaging, HCY induced a small NMDAR current on its own and reduced desensitization of the NMDAR response to glutamate. When HCY was washed out (washout peak normalized to initial control), sharp desensitization returned. B: when GLY (10 μM) and the GLY transporter antagonist NFPS (100 nM) were washed onto CA1, desensitization was progressively reduced in 3 of 4 cells tested. HCY had a strongly reduced effect in the presence of GLY and NFPS. (Note 1 μM strychnine is present to avoid GLY receptor activation by exogenously applied GLY.) C: box plots showing distributions of normalized charge transfer ratios in the presence and absence of GLY and NFPS.