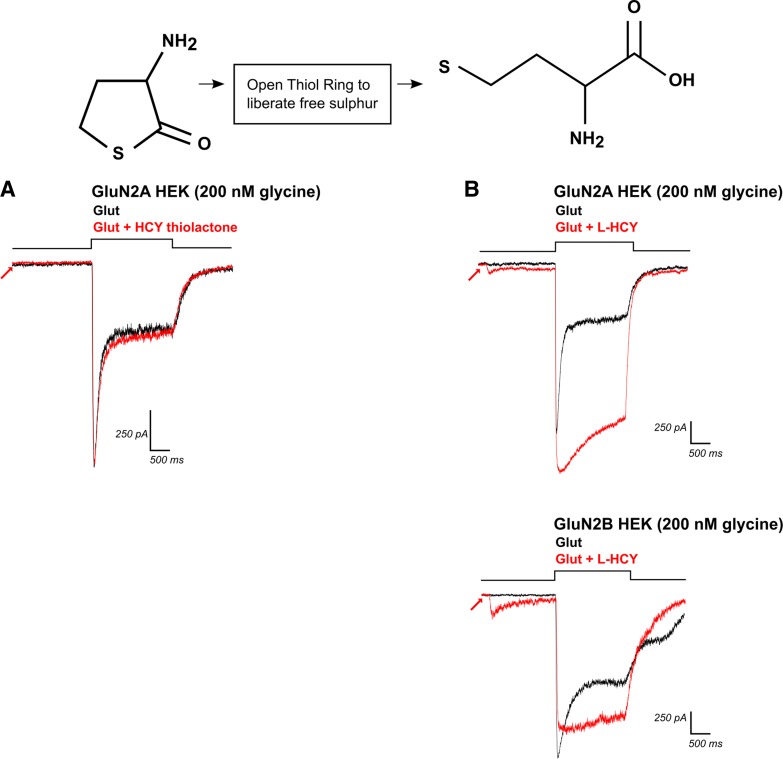
Fig. 7.
Opening the thiol ring of HCY-thiolactone creates l-HCY, which reproduces d,l-HCY effects. Top left: structure of HCY-thiolactone, with its sulfur group bound in a thiol ring. A: application of HCY-thiolactone (1 mM) did not significantly enhance charge transfer through NMDARs by glutamate (N = 5). B: opening the thiol ring of HCY-thiolactone creates l-HCY (see top right for structure). l-HCY showed effects that were qualitatively identical to d,l-HCY. l-HCY activated a small current on its own (see arrows), reduced desensitization, and enhanced peak amplitude of currents in GluN2A-transfected HEK cells (N = 3), while reducing peak amplitude and reducing desensitization of currents in GluN2B-transfected HEK cells (N = 3).