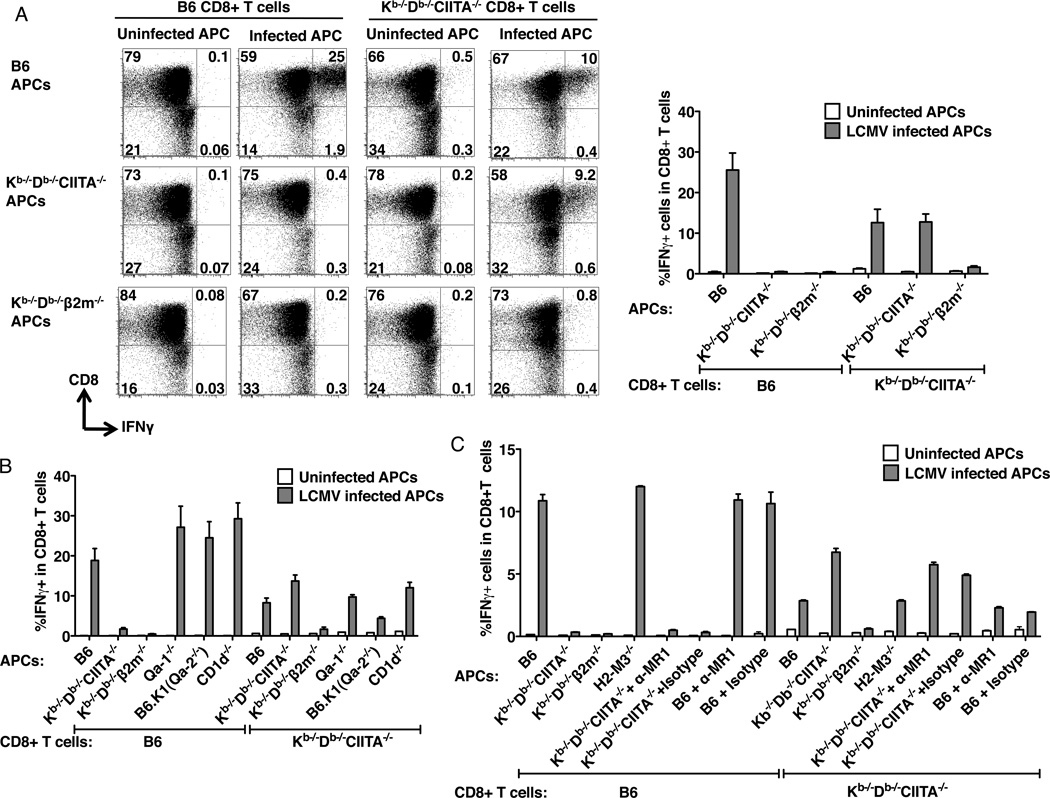
Figure 6.
MHC class Ib-restricted CD8+ T cells from LCMV-infected Kb−/−Db−/−CIITA−/− mice. (A) Peritoneal macrophages were harvested from ConA inoculated B6, Kb−/−Db−/−CIITA−/−, or Kb−/−Db−/−®2M−/− mice as a source of antigen presenting cells (APCs). APCs were either uninfected or infected with LCMV-Arm for 1 day. At day 8 post LCMV-infection, splenocytes were harvested from B6 or Kb−/−Db−/−CIITA−/− mice and CD8+ T cells were enriched and co-cultured with APC for 1 day, followed by analysis of IFN© production by flow cytometry. Left: Representative flow cytometry plots showing the production of IFN© by cultured CD8+ T cells. Right: The percentage of IFN©+ CD8+ T cells. T cells were obtained from 3 mice for each group. The data represent one of three independent experiments. (B) B6, Kb−/−Db−/−CIITA−/−, Kb−/−Db−/−β2m−/−, Qa-1−/−, B6.K1(Qa2−/−), and CD1d−/− bone marrow-derived macrophages were used as APCs. CD8+ T cells enriched from day 8 infected mice were co-cultured with the LCMV uninfected or infected APCs for 1 day, and the production of IFN© was measure by flow cytometry. (C) B6, Kb−/−Db−/−CIITA−/−, Kb−/−Db−/−β2m−/−, and H2-M3−/− bone marrow-derived macrophages were used as APCs. MR1 blocking antibody was added as indicated to the B6 or the Kb−/−Db−/−CIITA−/− APCs in 5µg/ml to block the MR1 molecule. CD8+ T cells enriched from day 8 infected mice were co-cultured with the LCMV uninfected or infected APCs for 1 day, and the production of IFNγ was measure by flow cytometry.