Abstract
Purpose
It is uncertain whether the tumor burden as assessed using FDG-PET has prognostic significance in newly diagnosed diffuse large B-cell lymphoma (DLBCL). The authors undertook this study to determine whether a parameter that reflects both FDG uptake magnitude and the greatest tumor diameter is a prognostic indicator in DLBCL.
Materials and Methods
Forty-two DLBCL patients (age, 57.4 ± 15.5 years; male/female = 25/17; stage I/II/III/IV=5/17/10/10) who underwent FDG-PET before chemotherapy were enrolled. A lesion with the highest maximum standardized uptake value (MaxSUV) on the PET image was selected, and size-incorporated MaxSUV (SIMaxSUV) of mass was calculated as MaxSUV × greatest diameter (mm) on the transaxial PET image. Median follow-up duration was 20.0 months.
Results
Twelve (28.6% = 12/42) patients experienced disease progression, and 10 (23.8% = 10/42) died during follow-up. Among six variables [Ann Arbor stage, %Ki-67 expression, International Prognostic Index (IPI), MaxSUV, greatest diameter, and SIMaxSUV] investigated, only SIMaxSUV was found to be a single determinant of progression-free and overall survivals by multivariate analyses (p < 0.05).
Conclusion
These results suggest that SIMaxSUV, a new FDG-PET parameter that incorporates FDG uptake magnitude and the greatest tumor diameter, may be a useful indicator of prognosis in untreated DLBCL.
Keywords: FDG-PET, Lymphoma, Prognosis, SUV, Size-incorporated maxSUV, Greatest diameter
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most prevalent type of non-Hodgkin’s lymphoma (NHL) [1]. Less than half of DLBCL patients are cured by standard treatment, and thus, it is essential that those requiring more aggressive therapy are identified [2].
Several prognostic models have been used for risk stratification in NHL. In particular, the International Prognostic Index (IPI) was designed to predict the risk of recurrence and overall survival in aggressive NHL [3]. The IPI is based on five pretreatment characteristics, i.e., age, Ann Arbor stage, number of extranodal sites, serum lactate dehydrogenase (LDH) levels, and performance status according to the Eastern Cooperative Oncology Group (ECOG) scale. However, the IPI does not provide a satisfactory explanation for the considerable survival variability associated with DLBCL [2].
Positron emission tomography (PET) using F-18 fluorodeoxyglucose (FDG) can provide quantitative information on the functional metabolic status in NHL. FDG-PET imaging after some cycles of chemotherapy may give prognostic information on recurrence and survival in lymphoma patients [4–7], and FDG uptake has been reported to be correlated with the histological grade of malignancy and proliferation rate in untreated NHL patients [8–11]. However, the role of FDG-PET in pretreatment risk stratification has not been investigated in a homogeneous group of DLBCL patients [12]. In addition to FDG uptake magnitude, PET can also measure the size of lesions with an abnormal metabolism.
This study was undertaken to assess the values of the FDG-PET parameters, i.e., maximum standardized uptake value (MaxSUV), greatest diameter, and size-incorporated MaxSUV (SIMaxSUV), as pretreatment prognostic indicators in DLBCL.
Materials and Methods
Patients
This study was retrospectively designed. Forty-two consecutive DLBCL patients who had been histologically confirmed but not treated were enrolled in the study from July 2003 to June 2006. Standard treatment and follow-up strategies were applied to all the patients. The disease progression was defined as new evidence of radiological or clinical alterations that led to the change of treatment strategy. Patients who had experienced FDG-PET only after treatment initiation and who had their follow-up record lost without any treatment were excluded from this study. The enrolled DLBCL patients underwent FDG-PET scanning within 1 month of starting induction of chemotherapy. This study was conducted in accord with institutional guidance for clinical studies.
FDG-PET
All patients were fasted for at least 6 h before FDG-PET whole-body scanning (Allegro, Philips Medical Systems, Cleveland, OH). F-18 FDG was intravenously injected at a dose of 5.18 MBq/kg (0.14 mCi/kg), and whole-body scanning was performed at 50 min after FDG injection from the skull base to upper thigh. Cs-137 transmission scans were obtained for attenuation correction. The 3D row-action maximum-likelihood algorithm was adopted for image reconstruction, and the resolution of the reconstructed trans-axial images was 4.8 mm. Glucose levels at FDG-PET acquisition were within the normal range in all patients 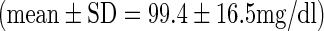 .
.
Lesion FDG uptake was measured using the standardized uptake value (SUV), which was defined as: radioactivity in ROI (Bq/ml) × lean body mass (kg)/injected radioactivity (Bq). Lean body masses were defined as: 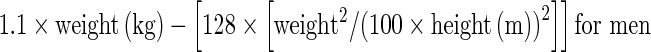 and
and 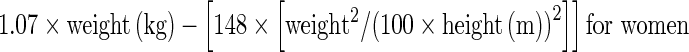 , which can be found at www.intmed.mcw.edu/clincalc/body.html. Regions of interest (ROIs) were drawn around lesions on trans-axial images. Of the lesions detected on whole-body PET images, the one with the highest maximum standardized uptake value (MaxSUV) was selected to measure the greatest diameter (mm) on PET transaxial images. The greatest diameter was measured in the same condition for the all lesions concerned. The upper window limit was set to the 50% level of its highest value, whereas the lower window limit remained fixed. The voxel size of the reconstructed whole-body image was
, which can be found at www.intmed.mcw.edu/clincalc/body.html. Regions of interest (ROIs) were drawn around lesions on trans-axial images. Of the lesions detected on whole-body PET images, the one with the highest maximum standardized uptake value (MaxSUV) was selected to measure the greatest diameter (mm) on PET transaxial images. The greatest diameter was measured in the same condition for the all lesions concerned. The upper window limit was set to the 50% level of its highest value, whereas the lower window limit remained fixed. The voxel size of the reconstructed whole-body image was 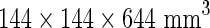 . The FDG-PET parameter size-incorporated MaxSUV (SIMaxSUV) was defined as MaxSUV × greatest diameter on the same lesion.
. The FDG-PET parameter size-incorporated MaxSUV (SIMaxSUV) was defined as MaxSUV × greatest diameter on the same lesion.
Statistical Analysis
Statistical analysis was performed using SPSS software (version 13.0). Survival time was defined as the time between the initial PET study and last follow-up. Six variables, including Ann Arbor stage, %Ki-67 expression as a marker of tumor proliferation, International Prognostic Index (IPI), MaxSUV, greatest diameter, and SIMaxSUV, were separately evaluated versus survival as continuous variables using Cox proportional hazard models. Then, the above-mentioned variables were stratified into two groups, i.e., Ann Arbor stage I, II versus III, IV; Ki-67 ≤90% versus >90%; the IPI 0, 1, 2 versus 3, 4, respectively. For survival analysis using the Kaplan-Meier log-rank test, MaxSUV, greatest diameter, and SIMaxSUV were repetitively dichotomized into two groups to identify the optimal dichotomizing condition with 1-unit increased intervals for MaxSUV, 10 units for greatest diameter, and 100 units for SIMaxSUV, respectively. Interactions between variables found to significantly affect survival time were evaluated by multivariate analysis using the Cox proportional hazard model. P values of less than 0.05 were considered significant.
Results
Patient Characteristics
Forty-two patients (25 men and 17 women) with DLBCL were analyzed during this study (Table 1). Mean patient age was 57.4 ± 15.5 years, range 21 to 82. Mean values and ranges of MaxSUV, greatest diameter, and SIMaxSUV are shown in Table 2. Median follow-up time was 20.0 months  .
.
Table 1.
Characteristics of the 42 diffuse large B-cell lymphoma patients
| Characteristics | No. of patients | % |
|---|---|---|
| Sex | ||
| Male | 25 | 59.5 |
| Female | 17 | 40.5 |
| Ann Arbor stage | ||
| 1 | 5 | 11.9 |
| 2 | 17 | 40.5 |
| 3 | 10 | 23.8 |
| 4 | 10 | 23.8 |
| IPIa | ||
| 0 | 7 | 16.7 |
| 1 | 9 | 21.4 |
| 2 | 12 | 28.6 |
| 3 | 8 | 19.0 |
| 4 | 6 | 14.3 |
aIPI: International Prognostic Index
Table 2.
Characteristics of diffuse large B-cell lymphoma lesions by FDG-PET
| Characteristics of lesions | Mean ± SD | Range |
|---|---|---|
| MaxSUVa | 10.2 ± 4.6 | 1.3 ∼ 21.2 |
| Greatest diameter (mm) | 43.0 ± 27.1 | 12 ∼ 124 |
| SIMaxSUVb | 465.9 ± 367.7 | 20.4 ∼ 1621.9 |
aMaxSUV: maximum standardized uptake value
bSIMaxSUV: size-incorporated maximum standardized uptake value (MaxSUV × greatest diameter)
Progression-free Survival Analysis
Twelve (28.6%) of the 42 patients experienced disease progression, and the median follow-up time to disease progression was 7.1 months  . Of these 12 patients, 6 experienced in situ recurrence and 6 distant metastases. No significant relation was found between recurrence types and the variables investigated (Ann Arbor stage, %Ki-67 expression, IPI, MaxSUV, greatest diameter, and SIMaxSUV).
. Of these 12 patients, 6 experienced in situ recurrence and 6 distant metastases. No significant relation was found between recurrence types and the variables investigated (Ann Arbor stage, %Ki-67 expression, IPI, MaxSUV, greatest diameter, and SIMaxSUV).
Univariate analysis revealed that Ann Arbor stage (I, II vs. III, IV), IPI (0, 1, 2 vs. 3, 4), MaxSUV (<11 vs. ≥11), greatest diameter (<50 mm vs. ≥50 mm), and SIMaxSUV (<500 vs. ≥500) were significantly correlated with PFS with p values of 0.001, 0.007, 0.020, 0.012, and 0.002, respectively (Table 3). However, %Ki-67 expression was not found to be related to PFS (p > 0.05). Combinatorial effects and interactions between variables that were significant by univariate analysis were examined using multivariate Cox proportional hazard models, and SIMaxSUV (<500 vs. ≥500) (p = 0.019) and Ann Arbor stage (I or II vs. III or IV) (p = 0.021) were found to be significantly associated with PFS (Table 4).
Table 3.
Univariate analysis of progression-free survival
| Variables | p-value |
|---|---|
| Ann Arbor stage (I, II vs. III, IV) | 0.001 |
| IPIa (0, 1, 2 vs. 3, 4) | 0.007 |
| %Ki-67 expression | 0.726 |
| MaxSUVb (<11 vs. ≥11) | 0.020 |
| Greatest diameter (<50 mm vs. ≥50 mm) | 0.012 |
| SIMaxSUVc (<500 vs. ≥500) | 0.002 |
aIPI: International Prognostic Index
bMaxSUV: maximum standardized uptake value
cSIMaxSUV: size-incorporated maximum standardized uptake value (MaxSUV × greatest diameter)
Table 4.
Multivariate analysis results of progression-free survival (Cox proportional hazard model)
| Variable | p-value | Relative risk | 95% CIb |
|---|---|---|---|
| SIMaxSUVa (<500 vs. ≥500) | 0.019 | 4.094 | 1.260–13.309 |
| Ann Arbor stage (I, II vs. III, IV) | 0.021 | 11.383 | 1.455–89.073 |
aSIMaxSUV: size-incorporated maximum standardized uptake value (MaxSUV × greatest diameter)
bCI: confidence interval
Overall Survival Analysis
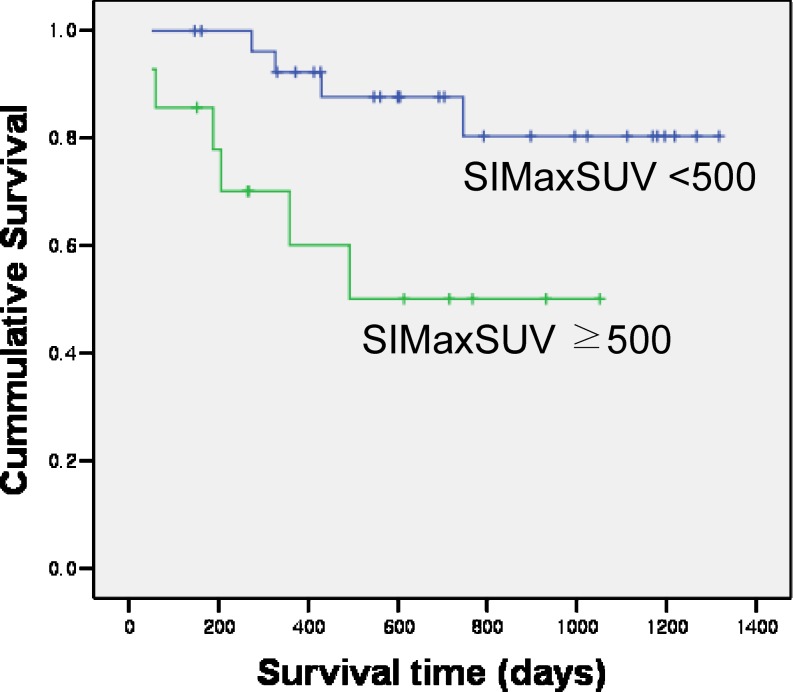
Ten (23.8%) of the 42 patients died during follow-up, and the median time to death was 10.0 months  . Univariate analysis revealed that IPI (0, 1, 2 vs. 3, 4), MaxSUV, greatest diameter (<50 mm vs. ≥50 mm), and SIMaxSUV were significantly related to overall survival with p values of 0.009, 0.007, <0.001, and <0.001, respectively (Table 5). Multivariate Cox proportional hazard analysis on IPI (0, 1, 2 vs. 3, 4), MaxSUV, greatest diameter (<50 mm vs. ≥50 mm), and SIMaxSUV revealed that only SIMaxSUV (p < 0.001) was significantly associated with overall survival (Table 6). The 14 patients with a SIMaxSUV ≥ 500 had a 1-year overall survival rate of 60.1%, which was significantly lower than a 1-year overall survival rate of 92.3% of the 28 patients with a SIMaxSUV of <500 (p < 0.05) (Fig. 1).
. Univariate analysis revealed that IPI (0, 1, 2 vs. 3, 4), MaxSUV, greatest diameter (<50 mm vs. ≥50 mm), and SIMaxSUV were significantly related to overall survival with p values of 0.009, 0.007, <0.001, and <0.001, respectively (Table 5). Multivariate Cox proportional hazard analysis on IPI (0, 1, 2 vs. 3, 4), MaxSUV, greatest diameter (<50 mm vs. ≥50 mm), and SIMaxSUV revealed that only SIMaxSUV (p < 0.001) was significantly associated with overall survival (Table 6). The 14 patients with a SIMaxSUV ≥ 500 had a 1-year overall survival rate of 60.1%, which was significantly lower than a 1-year overall survival rate of 92.3% of the 28 patients with a SIMaxSUV of <500 (p < 0.05) (Fig. 1).
Table 5.
Univariate analysis of overall survival
| Variables | p-value |
|---|---|
| Ann Arbor stage | >0.05 |
| IPIa (0,1, 2 vs. 3, 4) | 0.009 |
| %Ki-67 expression | >0.05 |
| MaxSUVb | 0.007 |
| Greatest diameter (<50 mm vs. ≥50 mm) | <0.001 |
| SIMaxSUVc | <0.001 |
aIPI: International Prognostic Index
bMaxSUV: maximum standardized uptake value
cSIMaxSUV: size-incorporated maximum standardized uptake value (MaxSUV × greatest diameter)
Table 6.
Multivariate analysis results of overall survival (Cox proportional hazard model)
| Variable | p-value | Relative risk | 95% CIb |
|---|---|---|---|
| SIMaxSUVa | <0.001 | 1.004 | 1.002–1.006 |
aSIMaxSUV: size-incorporated maximum standardized uptake value (MaxSUV × greatest diameter) as a continuous variable
bCI: confidence interval
Fig. 1.
Overall survival rate curves using a SIMaxSUV cutoff value of 500 are displayed. Patients with a SIMaxSUV of ≥500 (n = 14) had a lower 1-year overall survival rate (60.1%) than those with a SIMaxSUV of <500 (n = 28, 92.3%) (p < 0.05)
Discussion
The principal finding of this study is that tumor burden as assessed using SIMaxSUV, which reflects both the FDG uptake magnitude and greatest tumor diameter, is significantly correlated with prognosis in patients with non-treated DLBCL. SIMaxSUV was defined as the product of MaxSUV and the greatest diameter (mm), and was found to be the single most important determinant of disease progression and overall survival by multivariate analyses.
FDG uptake by lymphoma lesions might reflect the degree of aggressiveness. A high FDG accumulation in lymphoma has been associated with a high histological grade of malignancy [9–11, 13], and large lesions and bulky disease in NHL have been reported to be adverse prognostic factors in patients with aggressive NHL. Moreover, it was recommended that these factors be routinely considered in risk stratification to decide upon combined modality therapies [14–16]. The present study identified the MaxSUV and greatest diameter as prognostic factors in DLBCL by univariate analyses. However, the MaxSUV and greatest diameter were not prognostically significant according to multivariate analyses. The factor SIMaxSUV obtained by multiplying MaxSUV by the greatest diameter was found to be a better prognostic indicator than IPI, an established prognostic marker of DLBCL. Moreover, in this study, SIMaxSUV was found to be the only predictor of overall survival after adjusting for IPI, MaxSUV, and the greatest diameter. SIMaxSUV is composed of the highest FDG uptake, which represents the degree of glucose metabolic derangement, and the greatest diameter, which represents macroscopic growth of the DLBCL lesions. Thus, SIMaxSUV reflects both current metabolic activity and past proliferative action.
Another clinical implication of the present study is that FDG-PET performed before treatment could provide important prognostic information in cases of DLBCL. The major indication of FDG-PET in NHL was to differentiate between viable tumor tissue and fibrosis after chemotherapy, because complete remission after full cycles of chemotherapy is a determinant of cure in NHL [3, 17]. Thereafter, interim evaluation of response during chemotherapy has become a principal reason for conducting FDG-PET in DLBCL [18, 19]. The lack of data for prognostic value of pretreatment FDG-PET may lie in multiplicity of lymphoma lesions. It is difficult to identify a single representative lesion that determines the patient survival among multiple wide-spread lesions. MaxSUV of a single main lesion has been reported to be an independent prognostic marker of variable cancers [20–23], but in the case of DLBCL, the lesion with the greatest MaxSUV may determine patient survival only after tumor size information has been incorporated into the MaxSUV. A combination of the MaxSUV and greatest diameter has been suggested as a prognostic marker in newly diagnosed small cell lung cancer, another systemic malignant disease [24]. In this regard, the concept of the size incorporation into FDG uptake appears to be useful in terms of predicting the prognosis of inherently wide-spread malignant diseases such as DLBCL.
In conclusion, our results suggest that SIMaxSUV, which reflects both the FDG uptake magnitude and greatest tumor diameter, can be used as a prognostic indicator in DLBCL before treatment.
Limitation
The small number of enrolled cases and the shorter time of follow-up periods are major drawbacks of the present study. Recently, total lesion glycolysis, a product of meanSUV and lesion volume, was suggested as a prognostic marker of DLBCL patients who had been treated using radioimmunotherapy [25]. We did not evaluate the prognostic power of the meanSUV and lesion volume, but of the maxSUV and greatest diameter. Thus, further prospective multi-institutional studies are required in order for the SIMaxSUV to be accepted as a decisive prognostic marker of untreated DLBCL.
Footnotes
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund; KRF-2007-331-E00168).
References
- 1.Jhanwar YS, Straus DJ. The role of PET in lymphoma. J Nucl Med. 2006;47:1326–1334. [PubMed] [Google Scholar]
- 2.Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:995–1007. doi: 10.1200/JCO.2005.02.4786. [DOI] [PubMed] [Google Scholar]
- 3.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 4.Mikhaeel NG, Timothy AR, O’Doherty MJ, Hain S, Maisey MN. 18F-FDG-PET as a prognostic indicator in the treatment of aggressive non-Hodgkin’s lymphoma—comparison with CT. Leuk Lymphoma. 2000;39:543–553. doi: 10.3109/10428190009113384. [DOI] [PubMed] [Google Scholar]
- 5.Spaepen K, Stroobants S, Dupont P, Van Steenweghen S, Thomas J, Vandenberghe P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–419. doi: 10.1200/JCO.2001.19.2.414. [DOI] [PubMed] [Google Scholar]
- 6.Spaepen K, Stroobants S, Dupont P, Thomas J, Vandenberghe P, Balzarini J, et al. Can positron emission tomography with [(18)F]-fluorodeoxyglucose after first-line treatment distinguish Hodgkin’s disease patients who need additional therapy from others in whom additional therapy would mean avoidable toxicity? Br J Haematol. 2001;115:272–278. doi: 10.1046/j.1365-2141.2001.03169.x. [DOI] [PubMed] [Google Scholar]
- 7.Schot B, van Imhoff G, Pruim J, Sluiter W, Vaalburg W, Vellenga E. Predictive value of early 18F-fluoro-deoxyglucose positron emission tomography in chemosensitive relapsed lymphoma. Br J Haematol. 2003;123:282–287. doi: 10.1046/j.1365-2141.2003.04593.x. [DOI] [PubMed] [Google Scholar]
- 8.Okada J, Yoshikawa K, Itami M, Imaseki K, Uno K, Itami J, et al. Positron emission tomography using fluorine-18-fluorodeoxyglucose in malignant lymphoma: a comparison with proliferative activity. J Nucl Med. 1992;33:325–329. [PubMed] [Google Scholar]
- 9.Lapela M, Leskinen S, Minn HR, Lindholm P, Klemi PJ, Soderstrom KO, et al. Increased glucose metabolism in untreated non-Hodgkin’s lymphoma: a study with positron emission tomography and fluorine-18-fluorodeoxyglucose. Blood. 1995;86:3522–3527. [PubMed] [Google Scholar]
- 10.Rodriguez M, Rehn S, Ahlstrom H, Sundstrom C, Glimelius B. Predicting malignancy grade with PET in non-Hodgkin’s lymphoma. J Nucl Med. 1995;36:1790–1796. [PubMed] [Google Scholar]
- 11.Schoder H, Noy A, Gonen M, Weng L, Green D, Erdi YE, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:4643–4651. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 12.Israel O, Keidar Z, Bar-Shalom R. Positron emission tomography in the evaluation of lymphoma. Semin Nucl Med. 2004;34:166–179. doi: 10.1053/j.semnuclmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Leskinen-Kallio S, Ruotsalainen U, Nagren K, Teras M, Joensuu H. Uptake of carbon-11-methionine and fluorodeoxyglucose in non-Hodgkin’s lymphoma: a PET study. J Nucl Med. 1991;32:1211–1218. [PubMed] [Google Scholar]
- 14.Mackintosh JF, Cowan RA, Jones M, Harris M, Deakin DP, Crowther D. Prognostic factors in stage I and II high and intermediate grade non-Hodgkin’s lymphoma. Eur J Cancer Clin Oncol. 1988;24:1617–1622. doi: 10.1016/0277-5379(88)90054-5. [DOI] [PubMed] [Google Scholar]
- 15.Oguchi M, Ikeda H, Isobe K, Hirota S, Hasegawa M, Nakamura K, et al. Tumor bulk as a prognostic factor for the management of localized aggressive non-Hodgkin’s lymphoma: a survey of the Japan lymphoma radiation therapy group. Int J Radiat Oncol Biol Phys. 2000;48:161–168. doi: 10.1016/S0360-3016(00)00480-6. [DOI] [PubMed] [Google Scholar]
- 16.Wilder RB, Rodriguez MA, Ha CS, Pro B, Hess MA, Cabanillas F, et al. Bulky disease is an adverse prognostic factor in patients treated with chemotherapy comprised of cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy for aggressive lymphoma. Cancer. 2001;91:2440–2446. doi: 10.1002/1097-0142(20010615)91:12<2440::AID-CNCR1279>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Coiffier B. How to interpret the radiological abnormalities that persist after treatment in non-Hodgkin’s lymphoma patients? Ann Oncol. 1999;10:1141–1143. doi: 10.1023/A:1008308129857. [DOI] [PubMed] [Google Scholar]
- 18.Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–1381. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 19.Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–1523. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- 20.Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–3260. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- 21.Leboulleux S, Dromain C, Bonniaud G, Auperin A, Caillou B, Lumbroso J, et al. Diagnostic and prognostic value of 18-fluorodeoxyglucose positron emission tomography in adrenocortical carcinoma: a prospective comparison with computed tomography. J Clin Endocrinol Metab. 2006;91:920–925. doi: 10.1210/jc.2005-1540. [DOI] [PubMed] [Google Scholar]
- 22.Pryma DA, Schoder H, Gonen M, Robbins RJ, Larson SM, Yeung HW. Diagnostic accuracy and prognostic value of 18F-FDG PET in Hurthle cell thyroid cancer patients. J Nucl Med. 2006;47:1260–1266. [PubMed] [Google Scholar]
- 23.Nguyen XC, Lee WW, Chung JH, Park SY, Sung SW, Kim YK, et al. FDG uptake, glucose transporter type 1, and Ki-67 expressions in non-small-cell lung cancer: correlations and prognostic values. Eur J Radiol. 2007;62:214–219. doi: 10.1016/j.ejrad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Song YS, Lee WW, Chung J-H, Park SY, Kim YK, Kim SE (2007) Tumor burden assessed by maxSUV and metabolic size on FDG-PET predicts prognosis of small cell lung cancer. J Nucl Med [abstract]
- 25.Cazaentre T, Morschhauser F, Vermandel M, Betrouni N, Prangere T, Steinling M, et al. Pre-therapy (18)F-FDG PET quantitative parameters help in predicting the response to radioimmunotherapy in non-Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2009 doi: 10.1007/s00259-009-1275-x. [DOI] [PubMed] [Google Scholar]